Abstract
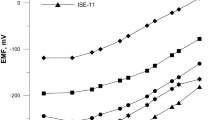
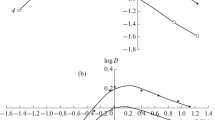
The manifestation of the ligand function of \({\text{Zn}}\left( {{\text{NCS}}} \right)_{4}^{{2 - }}{\text{-}}\) and \({\text{Co}}\left( {{\text{NCS}}} \right)_{4}^{{2 - }}{\text{-}}\)selective electrodes based on higher quaternary ammonium salts is explained by the exchange displacement of zinc and cobalt thiocyanates by SCN– ions from the membrane into the near-electrode layer of the solution. Its action is limited by the dissociation of the quaternary ammonium salt as an associate with \({\text{Zn}}\left( {{\text{NCS}}} \right)_{4}^{{2 - }}\) and \({\text{Co}}\left( {{\text{NCS}}} \right)_{4}^{{2 - }}.\) ions. The effect of the background concentration of CoCl2 or ZnCl2 on the selectivity of \({\text{Zn}}\left( {{\text{NCS}}} \right)_{4}^{{2 - }}{\text{-}}\) and \({\text{Co}}\left( {{\text{NCS}}} \right)_{4}^{{2 - }}{\text{-}}\)selective electrodes based on higher quaternary ammonium salts to SCN– ions is studied. The introduction of CoCl2 or ZnCl2 into the solution binds cobalt or zinc ions released from the membrane into thiocyanate complexes. The high selectivity of the \({\text{Zn}}\left( {{\text{NCS}}} \right)_{4}^{{2 - }}{\text{-}}\) and \({\text{Co}}\left( {{\text{NCS}}} \right)_{4}^{{2 - }}{\text{-}}\)selective electrodes to SCN– ions is due to the higher extraction ability of the cobalt and zinc complexes with SCN– ions compared to acid complexes with the competing anions. It is demonstrated that \({\text{Zn}}\left( {{\text{NCS}}} \right)_{4}^{{2 - }}{\text{-}}\) and \({\text{Co}}\left( {{\text{NCS}}} \right)_{4}^{{2 - }}{\text{-}}\)selective electrodes can be used for the determination of SCN– ions in process solutions in the production of polyacrylonitrile fibers.



Similar content being viewed by others
REFERENCES
Rakhman’ko, E.M., Doctoral (Chem.) Dissertation, Minsk: Belarusian State Univ., 1994.
Starobinets, G.L., Rakhman’ko, E.M., and Lomako, V.L., Zh. Anal. Khim., 1981, vol. 36, no. 7, p. 1305.
Rakhman’ko, E.M., Lomako, V.L., Poklonskaya, T.E., Kachanovich, I.V., and Serdyukova, I.E., Zh. Anal. Khim., 1995, vol. 50, no. 2, p. 200.
Rakhman’ko, E.M., Lomako, S.V., and Lomako, V.L., J. Anal. Chem., 2001, vol. 56, no. 10, p. 957.
Rakhman’ko, E.M., Lomako, S.V., and Lomako, V.L., J. Anal. Chem., 2000, vol. 55, no. 4, p. 363.
Aslan, N., Kenar, A., Atakol, O., and Kiliç, E., Erciyes Univ. Fen Bilimleri Enst. Derg., 2009, vol. 25, nos. 1–2, p. 237.
Ganjali, M.R., Norouzi, P., Faridbod, F., and Pourjavid, M.R., J. Iran. Chem. Soc., 2007, vol. 4, no. 1, p. 1.
Wen-Ju Xu, Ya-Qin Chai, Ruo Yuan, and Su-Li Liu, Anal. Bioanal. Chem., 2006, vol. 385, p. 926.
Won-Sik Han, Tae-Kee Hong, and Young-Hoon Lee, Am. J. Anal. Chem., 2011, vol. 2, p. 731.
Benvidi, A., Ghanbarzadeh, M.T., Dehghan, M., Mazloum-Ardakani, M., and Vafazadeh, R., Chin. Chem. Lett., 2014, vol. 25, no. 12, p. 1639.
Xie, W.J. and Gao, Y.Q., J. Phys. Chem. Lett., 2013, vol. 4, p. 4247.
Egorov, V.V., Rakhman’ko, E.M., and Rat’ko, A.A., Anion-selective electrodes with liquid membranes, in Encyclopedia of Sensors, Stevenson Ranch, CA: Am. Sci. Publ., 2006, vol. 1, p. 211.
Starobinets, G.L., Rakhman’ko, E.M., and Lomako, V.L., Zh. Anal. Khim., 1981, vol. 36, no. 7, p. 1305.
Koryta, J. and Stulic, K., Iontove-Selektivni Elektrody (Ion-Selective Electrodes), Prague: Academia, 1984.
Rakhman’ko, E.M., Matveichuk, Yu.V., and Yasinetskii, V.V., Vestn. Nats. Akad. Nauk Belarusi, Ser. Khim. Nauki, 2012, no. 2, p. 47.
Matveichuk, Yu.V., Rakhman’ko, E.M., Yasinetskii, V.V., and Stanishevskii, L.S., Metody Ob”ekty Khim. Anal., 2012, vol. 7, no. 4, p. 164.
Egorov, V.V., Rakhman’ko, E.M., Gulevich, A.L., Lomako, S.V., and Rat’ko, A.A., Russ. J. Coord. Chem., 2002, vol. 28, no. 10, p. 709.
Rakhman’ko, E.M., Matveichuk, Yu.V., and Kachanovich, I.V., Rodanidnye kompleksy metallov v ekstraktsii i ionometrii (Thiocyanate Metal Complexes in Extraction and Potentiometry), Minsk: Belarus Gos. Univ., 2017.
Rakhman’ko, E.M., Matveichuk, Yu.V., and Yasinetskii, V.V., Vestn. Nats. Akad. Nauk Belarusi, Ser. Khim. Nauki, 2012, no. 3, p. 54.
Rabinovich, V.A. and Khavin, Z.Ya., Kratkii khimicheskii spravochnik (Brief Handbook on Chemistry), St. Petersburd: Khimiya, 1994.
Spravochnik khimika (Chemist Handbook), 6 vols., Leningrad: Khimiya, 1965, vol. 3.
Kratkii spravochnik po khimii (Short Guide to Chemistry), Kurilenko, O.D., Ed., Kiev: Naukova Dumka, 1974.
Lur’e, Yu.Yu., Spravochnik po analiticheskoi khimii (Handbook on Analytical Chemistry), Moscow: Khimiya, 1989.
Williams, W.J., Handbook of Anion Determination, London: Butterworth, 1979.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Zhukova
Rights and permissions
About this article
Cite this article
Matveichuk, Y.V., Rakhman’ko, E.M. Ligand Function of Ion-Selective Electrodes Reversible to Zinc and Cobalt Thiocyanate Complexes: Causes of Formation, Mathematical Description, and Analytical Applications. J Anal Chem 74, 715–721 (2019). https://doi.org/10.1134/S106193481905006X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106193481905006X