Abstract
The unique properties of metal nanoparticles (NPs) resulting from their localized surface plasmon resonance have led to the emergence and rapid development of promising scientific areas. One of these areas is thermoplasmonics, which is based on the ability of such NPs to effectively transform optical radiation into heat. We discuss the optical properties of noble metal NPs, the main approaches to their synthesis, as well as the latest advances of thermoplasmonics in the field of biomedicine. The focus of this review is on photothermal diagnostics and therapy (theranostics) of various diseases. Note that, in addition to theranostics of tumors, the prospects for the use of plasmonic NPs in cardiology, ophthalmology, the fight against bacterial and viral infections, and other biomedical fields have been analyzed.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
Localized surface plasmon resonance (LSPR), which is inherent in metal nanoparticles (NPs), and the broad practical potentials associated with it are among the “hottest” topics that are discussed in the scientific and technical community for the past 20−30 years. This phenomenon arises when the oscillation frequency of conduction electrons in NPs coincides with the frequency of an incident radiation and manifests itself as an intense peak in the particle extinction spectrum.
Within the framework of the Mie theory, which describes the interaction of metal NPs with electromagnetic radiation, total extinction cross section Cext of an individual NP is the sum of the contributions from surface plasmon absorption Cabs and scattering Csca [1]. The resonant enhancement of these characteristics of the LSPR band leads to the fact that the efficiencies of particle scattering Qsca and absorption Qabs of radiation with a given resonant wavelength calculated as the ratios of Csca and Cabs to the geometric cross section of an NP can reach values of ten and more. This fact is of fundamental importance for solving various practical problems [2–4]. For example, the ability of plasmonic NPs to scatter radiation, as well as to enhance the electromagnetic field in the immediate vicinity of their surface, not only opens up the possibility of creating highly sensitive sensors, light-emitting devices, and diagnostic tools for various dangerous diseases [2–4], but also makes it possible to control optical radiation fluxes at the nanolevel [5]. At the same time, the absorption of radiation with a wavelength corresponding to LSPR by metal NPs leads to their strong heating. This phenomenon has led to the emergence of thermoplasmonics, a new field of research that includes the use of nanoparticles in biomedicine, nanofluidics, catalysis, and other fields of science and technology. In the last decade, this field is intensively developed. This is evidenced by a drastic increase in the number of experimental works on the analysis of the photothermal efficiency (PTE) of plasmonic particles and the search for new ways of their application, as well as the appearance of monographs and reviews devoted to this scope in leading scientific journals [6–9]. Note that, as a rule, these reviews discuss the use of gold NPs, while much less attention has been paid to silver NPs.
In this review, along with a brief analysis of the data relevant to the regulation of the morphology, optical characteristics, and photothermal efficiency of Au and Ag NPs, the latest advances of the thermoplasmonics in the field of treating various dangerous diseases will be discussed and the most promising applications using particles of these two metals will be indicated.
To obtain a sufficiently complete picture, let us, first of all, turn to the fundamental ideas of the regularities in the behavior of plasmonic NPs when they absorb laser radiation and the main factors that determine the PTE of particles.
2 GENERAL CONCEPTS OF THE BEHAVIOR OF PLASMONIC NANOPARTICLES UNDER THE ACTION OF LASER RADIATION
It should be emphasized that most of the works devoted to studying the heating of plasmonic NPs under laser radiation were carried out using gold NPs as models. This is largely due to the fact that such particles are intensively studied as thermosensitizers for laser hyperthermia of malignant neoplasms (see, e.g., [7–10]). Naturally, all regularities found for gold NPs are equally applicable to NPs of other metals.
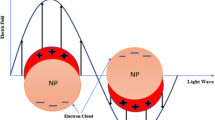
When plasmonic NPs are exposed to laser radiation, the generation wavelength of which coincides with the resonance frequency, photon energy is partly absorbed by conduction electrons (Fig. 1), thus leading to a dramatic increase in their kinetic energy [8, 9, 11, 12]. These high-energy “hot” electrons, which primordially have a nonequilibrium energy distribution, relax due to collisions with “cold” electrons belonging the conduction band; the characteristic relaxation time is, in this case, 10−100 fs [9, 12]. At this stage, the energy exchange between free electrons and an ion matrix (“lattice”) is actually absent. As a consequence, temperature Te of the electron gas may amount to from several hundred to several thousand of Kelvins, depending on the conditions of laser irradiation, while the temperature of the ion lattice (Tl) remains unchanged.
The main stages of transformation of radiation absorbed by plasmonic NPs into heat [10].
Subsequently, the particle itself is heated due to the interaction of electrons with ions of the crystal lattice. The rate of this process is almost independent of NP sizes (with the exception of particles whose diameter is less than 5 nm), and their heating time is 2−50 ps, depending on initial Te [13, 14]. At this stage, an equilibrium temperature is reached inside of an NP (Te = Tl), while the ambient temperature remains unchanged and equal to its initial value.
At the final stage, NPs are cooled due to heat exchange with an environment. The characteristic time required to establish equilibrium at the particle–environment interface depends on the NP size, the nature of the environment, and the laser irradiation conditions. For example, in aqueous dispersions, it can vary from 100 ps to 1 ns [13]. An increase in the temperature in the immediate vicinity of NPs may be as high as several tens or several hundreds of Kelvins and accompanied by the occurrence of certain processes in the environment [15–17].
For example, the results of the simulating the time evolution of temperature inside of a gold NP (with diameter d = 55 nm) and in its aqueous environment upon absorption of a femtosecond laser pulse with a generation wavelength of 400 nm have indicated that, under such conditions, the electron temperature reaches approximately 8000 K, while the temperature of the ion lattice is about 3000 K. The aqueous environment in the immediate vicinity of the NP is heated up to ≈550 K, thereby leading to its instant (“explosive”) evaporation with the formation of vapor bubbles. The growth or collapse of these bubbles may lead to the generation of acoustic (and, in some cases, shock) waves in the environment [15]. (Note that the mechanism of explosive water evaporation is actively discussed in relation to the use of plasmonic NPs for the photothermal therapy of malignant neoplasms [15, 18, 19].)
Laser-induced heating of NPs and their cooling due to heat exchange with the environment are, naturally, competing processes. If the heating rate significantly exceeds the cooling rate, the excess heat accumulated in an NP causes its melting, as well as the occurrence of other processes, such as NP evaporation, plasma generation, and fragmentation (“Coulomb explosion”), which may be accompanied by the appearance of shock waves in the environment. The regularities of these processes have been considered in detail in reviews [11, 15, 18, 20].
To conclude this section, it should be emphasized that there are two possible modes of laser-induced heating of NPs. The first of them is realized under the action of cw laser radiation, when the exposure time is much longer than the heat transfer time, while the second one results from pulsed irradiation, when these times are comparable. Both variants are actively used both in laboratory experiments and in practical applications. However, the mechanisms that determine the occurrence of a particular process, and, as a consequence, the efficiencies of NP use may be significantly different.
3 INTERRELATION OF MORPHOLOGY, PLASMON CHARACTERISTICS, AND PHOTOTHERMAL EFFICIENCY OF NANOPARTICLES
The photothermal efficiency of NPs, i.e. their ability to transform laser radiation into heat, depends on their material, size, and structure [21–33]. According to theoretical estimates, one of the most promising materials in this regard is silver [22–25]. This is indicated by its significantly higher (compared to gold) values of Qabs and Jolie number, which characterizes the ability of NPs to generate heat under irradiation at a wavelength corresponding to the LSPR maximum [22, 34]. However, it should be taken into account that, due to the high reactivity of this metal, the surface of NPs can be oxidized and, as a result, their PTE may be decreased.
Variations in the size, shape, and structure of NPs make it possible to control not only the position of the plasmon peak, thereby tuning it to a desired wavelength, but also the ratio between the scattering and absorption contributions to the particle extinction spectrum.
3.1 Effects of the Nature and Morphology of NPs on Their Plasmon Characteristics
An increase in the size of spherical NPs leads to a bathochromic shift of the LSPR position [26, 27]; however, this shift does not exceed 150−200 nm. For example, the plasmon peak of gold NPs shifts from nearly 520 to 700−750 nm with an increase in their diameter from approximately 10 to 180 nm [26]. The LSPR shift is accompanied by a gradual broadening of the plasmon peak, and, then, by its splitting due to the excitation of higher-order resonances in particles larger than the electron mean free path. For example, a quadrupole resonance, whose maximum is located near 550 nm, may be excited. A similar situation is also observed for spherical silver particles [27]: as their size increases, the LSPR shifts from 400 to ≈600 nm. The ability of NPs to absorb and scatter incident radiation increases with their sizes [11]; however, the contribution of scattering to the extinction spectrum also increases significantly, thus leading to a decrease in the PTE of the particles (see below).
For particles with a more complex structure and/or shape, a significant bathochromic shift of the LSPR relative to the position characteristic of spheres is observed. As an example, Fig. 2 shows the spectra of aqueous dispersions of the main types of gold NPs, which are characterized by LSPR variable within a wide range, as well as their micrographs taken by transmission or scanning electron microscopy. Sometimes, several peaks are distinctly seen in the spectra. In particular, two resonance peaks are observed for gold nanorods (GNRs) (Fig. 2c). The first one with λmax at nearly 500 nm corresponds to electron oscillations directed transverse to a nanorod, while the second one in the region of larger λ values is attributed to longitudinal oscillations. The position of the longitudinal resonance strongly depends on nanorod axial ratio (i.e., on the ratio between GNR length and diameter) and shifts to the red region as this ratio increases (Fig. 3). Note that, for star-shaped and prismatic NPs, the bathochromic shift of LSPR also increases with the degree of particle anisotropy (Figs. 2a, 2b).
Typical dependence of the position of the longitudinal LSPR of gold nanorods on their axial ratio [30].
In the case of metal nanoshells (both hollow and formed on cores of different natures), the reason for the LSPR shift is different. The optical properties of such NPs are most adequately described by the plasmon hybridization theory, which is a kind of an analog of the theory of molecular orbitals [31, 35–37]. Within the framework of this theory, the resonance properties of a metal nanoshell can be considered to be a result of the interaction (“hybridization”) of plasmons on its outer and inner surfaces. In the case of spherical particles, such hybridization leads to the appearance of two plasmon resonances. The first of them is symmetric or “bonding” (by analogy with the bonding molecular orbital), is characterized by a lower energy and a higher dipole moment; it strongly interacts with the incident radiation. It is this resonance that is responsible for the appearance of the intense absorption band in the long-wavelength region of the spectrum. The second resonance is asymmetric (“antibonding”) and has a higher energy; however, it rather weakly interacts with the incident radiation. As a consequence, the short-wavelength peak inherent in this resonance is very poorly pronounced in the spectrum of nanoshells (in the case of gold shells, it is completely hidden by the region of interband transitions). The degree of hybridization of plasmons depends on their energies on the outer and inner surfaces of the shell and its thickness [35, 36]. This theory can also be employed to describe the resonances of nanoshells having other shapes; however, the hybridization pattern becomes, in this case, more complex [7, 31, 36, 37]. For example, the LSPR of a spindle-shaped nanoshell is represented to be a result of hybridization of plasmons of a solid metal ellipsoid and a cavity of the same size as that of a “core” in a bulk metal. Such structures are characterized by the existence of two resonances, transverse and longitudinal, with their positions depending on the ratio between the length of the ellipsoid and its maximum diameter [31, 36].
The LSPR of nanoshells is tuned to a preset wavelength by varying the ratio of the characteristic size of the “core” (or the internal cavity) to the shell thickness. The larger this ratio, the greater the bathochromic shift of the LSPR relative to its position inherent in spheres (Figs. 2d−2f). In turn, the quality factor of the LSPR and the ratio between the scattering and absorption cross sections of such particles strongly depend on not only the above ratio, but also the particle size as a whole [38–40]. An increase in the size leads to a rise in the contribution of scattering and broadening of the plasmon peak. The latter phenomenon is due to the appearance of higher-order resonances and a phase shift that occurs when the particle size becomes comparable with the wavelength of the incident radiation [41].
The plasmonic properties of GNRs and spherical SiO2@Au core/shell shell particles were systematically compared with the properties of Au nanospheres in [39]. In the case of GNRs, the authors used the concept of the effective NP radius (Reff), which was equal to radius R of a spherical NP with the same volume as the volume of the nanorod. It was calculated by equation
where V is the particle volume. This made it possible to compare the efficiencies of radiation scattering and absorption by particles of different shapes.
In [39], the main attention was focused on plasmonic NPs, whose LSPR maximum was in the near infrared region (λmax = 800−1100 nm). It is this range that corresponds to the maximum transmission of biological tissues and attracts the greatest interest as applied to the biomedical use of NPs. Calculations have shown that the Qext, Qabs, and Qsca values at the LSPR maximum for nanorods are significantly higher than those for spherical NPs and core/shell particles. As Reff grows, the ability of GNRs to scatter and absorb incident radiation, as well as the relative contribution of scattering to the extinction spectrum, increase.Footnote 1 The value of this contribution depends on both the volume of GNRs and their diameter at a constant volume [39]. As will be shown below, variations in the Qabs-to-Qsca ratio significantly affects the PTE of particles.
3.2 Synthesis of NPs with a Specified Position of LSPR
NPs with a required morphology are obtained using colloidal methods based on reducing ions of the corresponding metals in liquid (most often, aqueous) media [26, 27, 42–59]. As a rule, organic compounds that act as stabilizers of NPs being formed are additionally introduced into a reaction mixture. These stabilizers can not only provide NPs with required sizes, but also govern their shape due to the specific adsorption on these or those crystallographic faces of growing NPs.
The simplest task is the synthesis of spherical gold NPs [26, 42]. The main method of their preparation is the so-called “citrate” synthesis, which involves the use of sodium citrate as a reductant of Au(3+) ions. It has been shown (see review [42] and references therein) that, by varying the precursor/reductant ratio, it is possible to obtain almost monodisperse NPs with diameters from ≈10 to ≈100 nm. Moreover, the introduction of additional portions of a precursor and a reductant into a system makes it possible to increase the NP sizes to 200−300 nm [26].
As regards the preparation of NPs with LSPR positions tunable in a wide range, this problem can be believed to be completely solved only as applied to gold NPs largely due to the high chemical inertness of gold. To date, a sufficient number of reproducible and scalable methods have been developed for producing not only NPs of different sizes and shapes, but also composite structures with dielectric cores and gold shells. These methods have been analyzed in detail in relatively recent reviews [42, 45, 47].
As a first approximation, they can be divided into two groups. One of them includes methods based on the growth (enlargement) of ultrasmall spherical gold NPs (so-called seed NPs) in an aqueous or nonaqueous solution containing gold ions, a mild reductant, and, in some cases, additives that promote an anisotropic growth of particles. These methods are actively used both for the synthesis of anisotropic NPs of various shapes (star-shaped, prismatic, or rodlike) and for the formation of gold nanoshells on the surfaces of spherical or anisotropic nanocores (for example, of silica or iron oxideFootnote 2) [31, 32, 42–45]. Their common essential disadvantage is the high sensitivity of the process to the quality of seed particles [42, 45–47]. That is why the so-called seedless (or one-pot) methods for the synthesis of star-shaped and rodlike Au NPs have recently appeared and begun to gain popularity [30, 42, 48]. In addition to the easiness of realization, these methods make it possible to obtain small particles characterized by increased PTE.
The second of the aforementioned groups includes methods based on the galvanic replacement of a less noble metal (for example, silver) with a more noble one, e.g., gold, and makes it possible to form hollow nanoshells of spherical, cubic, and other shapes [42, 49–52]. For this purpose, a solution of chloroauric acid is added to a colloidal solution containing Ag NPs of this or that shape. The process is described by the following scheme:
Such a process can proceed in the absence of a reductant; however, according to the data of some authors (see, e.g., [51]), its introduction enables one to better control the reduction rate of gold ions and obtain more uniform hollow NPs. As has been shown in [52] using the synthesis of gold nanocages as an example, the introduction of cetyltrimethylammonium chloride into a reaction mixture also leads to a similar result.
It should be emphasized that a variation in the size, shape, and structure (i.e., the position of the LSPR) of Ag NPs is a much more difficult task. The analysis of the literature data [53–59] has shown that almost all methods proposed for the synthesis of nonspherical Ag NPs are not only poorly scalable, but also very sensitive to the purity of the used reagents.
The most common method for obtaining anisotropic silver particles is the so-called polyol synthesis, which is based on the reduction of metal ions in the media of polyatomic alcohols in the presence of poly(vinylpyrrolidone) used as a stabilizer (see, e.g., review [58] and references therein). However, it should be taken into account that the size of the particles formed in this case is, as a rule, rather large. This leads to a significant prevalence of Qsca over Qabs and makes the use of such particles in thermoplasmonics inefficient. Actually, the only exception is silver nanocubes. For example, the authors of [56, 60] showed, in particular, the possibility of obtaining monodisperse silver nanocubes with face sizes of 30−60 nm, which were then used as “sacrificial” (i.e., soluble) templates in the synthesis of gold nanocages. Then, it was shown that such cubic silver NPs could also be obtained in an aqueous medium [52]. In this case, cetyltrimethylammonium chloride served as a stabilizer for the particles being formed.
3.3 Photothermal Efficiency of Plasmonic NPs of Different Morphologies
Despite the fact that, the LSPR of diverse particles is actually in the same spectral range, the Q-factors of their resonance peaks differ significantly. The same can be said about the contributions of absorption and scattering to the extinction spectra of NPs, as well as about the efficiency of the transformation of laser radiation energy into thermal energy. (Note that, when continuous radiation is used, this efficiency is governed by the specific absorption of a colloid.)
To date, rather many works have been published devoted to the theoretical and experimental evaluation of the PTE of plasmonic NPs (see, e.g., [21–33, 61–69]); however, it is extremely difficult to quantitatively compare the results obtained in these works because of different experimental conditions, measurement methods, and approaches used by the authors for data processing. It should be emphasized that the overwhelming majority of these studies were performed using gold NPs of various sizes and shapes. To a high extent, this is because it is difficult to obtain Ag NPs, whose LSPR position can be tuned in the near-IR region, which is of the greatest interest from the viewpoint of biomedicine.
Table 1 shows data on the PTEs of several main types of plasmonic NPs exposed to laser radiation at λmax ≈ 800 nm. It can be seen that the ability of metal NPs to transform incident radiation into thermal energy is, on the whole, noticeably higher than that of composite particles with a core/shell structure. Some exceptions are the so-called nanomatryoshkas, i.e., spherical particles with a gold core and alternating concentric shells of silica and gold. Due to the strong hybridization between the plasmons of the core and the gold shell, such particles very effectively absorb incident radiation [66].
Among the particles listed in Table. 1, the highest efficiency is inherent in the smallest GNRs, which is quite expected taking into account the estimations performed in [39]. An increase in the size of the GNRs (i.e., Reff) leads to a decrease in their ability to transform incident radiation into heat, because of a rise in the relative contribution of scattering to the extinction spectrum (as has already been noted above, this tendency is typical for particles of all types).
The higher Qabs values of silver than those of gold must promote a sufficiently high PTE of particles based on this metal. According to our data, for SiO2@Ag core/shell structures, it turns out to be somewhat higher than that recorded for GNRs. It is seen in Fig. 4 that the GNR dispersion is heated under the near IR laser irradiation at a higher rate than is the dispersion of SiO2/Ag particles; however, the temperature reached in this case is approximately 5°C lower.Footnote 3
Returning to the photothermal efficiency of GNRs, it should be emphasized that it depends significantly on the orientation of the particles in the electromagnetic field of an incident wave and increases when they are oriented along the field [13, 62]. It should be taken into account also that GNRs quite easily change their shape under the action of laser radiation; a similar situation is observed for gold nanostars and nanocubes. This process, whose features have been discussed in detail in reviews [7, 8, 11], is based on melting (and, in some cases, fragmentation) of particles under the action of high-energy laser pulses. This leads to a decrease in the axial ratio of NPs and a strong hypsochromic shift in their LSPR (up to 520−530 nm), thereby significantly reducing the absorption efficiency of near-IR radiation. The creation of semiconductor or dielectric shells on the surface of NPs hinders their transformation [70, 71]. Nevertheless, as the duration and/or energy of laser irradiation increases, both core and shells are gradually destroyed. As has been shown relatively recently, the latter process may be rather intense even under the irradiation with a low-power cw laser [72].
Note that, in some cases, the shells contribute to an increase in the PTE of particles [61, 73]. For example, according to [61], the presence of semiconductor shells of zinc or silver sulfides on the surface of spherical gold NPs leads to a bathochromic shift of their LSPR (from 500 to 800 nm) due to a change in the permittivity near the particle surface. As a consequence, the heating temperature of the corresponding dispersions under the action of a laser operating at a wavelength of 808 nm increases by several times. A similar effect of increasing PTE is also observed for gold NPs coated with graphene oxide, copper sulfide, or a layer of indocyanine green dye [73].
At the same time, for particles with a dielectric silica shell, the results are rather contradictory. For example, according to the estimates of the authors of [68], the PTE of nanorods coated with such a shell is 18.56%, which is noticeably lower than that recorded for “bare” GNRs of similar sizes (Table 1). However, the results of our experiments have indicated that the presence of an organosilica shell on the surface of GNRs has almost no effect on the heating kinetics of the corresponding dispersion and the maximum attainable temperature [74].
4 GOLD AND SILVER NANOPARTICLES IN THERMOPLASMONICS
Noble metal nanoparticles are among the most popular objects for thermoplasmonics and are used to solve a wide range of problems, including photothermal catalysis [8, 75–77], solar light harvesting [8, 78–80], laser ignition of energetic materials [81–85], various biomedical applications, etc. [6–9].
As has been noted above, the main goal of this review is to analyze the state of the art in the field of biomedical applications of thermoplasmonics and, first of all, its application for the treatment of various diseases.
4.1 Photothermal Therapy of Tumors
The possibility of tumor destruction due to the local heating under laser irradiation of SiO2@Au core/shell composite plasmonic particles introduced into itFootnote 4 was for the first time shown by Prof. Halas et al. in 2003 [86].
The success of these experiments induced the emergence and rapid development of one of the most urgent areas of thermoplasmonics, i.e., photothermal theranostics, which comprises both photothermal therapy (PTT) of malignant neoplasms (in this review, we will focus on this therapy) and the methods for their diagnosis based on the interaction of plasmonic NPs with laser radiation [45, 87]. A rather detailed analysis of these methods (first of all, optical coherence and photoacoustic tomography) was carried out simultaneously in several recent reviews (see, e.g., [7, 45, 54, 87]).
Along with the ability to transform the energy of absorbed radiation into heat, thus causing the death of tumor cells due to necrosis or apoptosis, when being intravenously administrated, plasmonic NPs can be selectively accumulated in tumors by the mechanisms of “passive” or “active” targeted delivery.
The first of them, which is referred to as the effect of enhanced permeability and retention, is based on the increased vascularization and permeability of the endothelium of blood vessels and capillaries in a tumor as compared with their endothelium in healthy tissues, as well as on the defectiveness of the lymphatic system of the tumor [38, 88]. In turn, the active transport of NPs is provided by their functionalization with biologically active molecules (antibodies, aptamers, folic acid, etc.) that are capable of identifying in this or that way tumor cells or elements of cell membranes, as well as by delivering the particles using certain types of cells (macrophages, leukocytes, erythrocytes, etc.) [88]. In particular, it has been shown [89] that the incorporation of gold NPs into mesenchymal stem cells leads to a great (37-fold) increase in the content of particles in a tumor and, hence, to a more pronounced therapeutic effect.
To date, a lot of experimental data have been collected on the PTT of tumors [42, 45, 87–102], with these data indicating the promise of this approach, as well as the possibility of its combination with chemo-, photodynamic, radiation, and other types of therapy.Footnote 5 Of greatest interest is the use of PTT to suppress tumor metastasis [91]. El-Sayed et al. were among the first to demonstrate the possibility of such suppression by the PTT of breast cancer in cats and dogs [92]. The mechanism of the observed effect remains to be clarified; however, according to the authors’ opinion, one of its reasons may be remodeling of the cytoskeleton of cancer cells and a decrease in their mobility [91, 93].
The combination of PTT with immunotherapy also makes it possible to inhibit the formation of metastases [42, 91, 94]. For example, it has been shown that PTT using gold nanostars followed by administration of PD-L1 antibodies facilitates not only to a significant increase in the efficiency of glioblastoma treatment, but also prevents the reappearance of this tumor [94].
The overwhelming majority of the studies devoted to PTT of tumors have been performed using gold NPsFootnote 6 and, primarily, GNRs (see, e.g., [87–99]), while silver particles have been used relatively rarely [69, 100–103]. This is due to both the difficulty of obtaining silver NPs with an LSPR position tunable in the near-IR region and the generally accepted opinion about the significantly higher toxicity of this metal.Footnote 7 The ability of this metal to induce the formation of reactive oxygen species is considered to be one of the main reasons for this toxicity [104].
In our opinion, the negative attitude to silver NPs, which is based on the idea of its toxicity, needs to be seriously rethought. For example, their ability to produce reactive oxygen species, which cause oxidative stress, can lead to the activation of the innate immune system and increase the efficiency of antitumor therapy. This is evidenced, in particular, by the results of works [105, 106] devoted to the use of silver NPs in the treatment of leukemia and melanoma. It has also been shown that silver NPs exhibit selective cytotoxicity towards cells of triple negative breast cancer, which is the most aggressive form of this disease [107]. This contributes to a decrease in their survival both under irradiation [107] and during combined radiation and photothermal therapy [102, 107]; in the latter case, silver nanoprisms with the LSPR maximum at nearly 800 nm were used in the experiments.
In addition, according to our data [101], composite particles with a silver shell provide a higher antitumor effect (compared to similar structures based on Au) during the PTT of S-57 sarcoma in mice using a pulsed laser operating at a wavelength of 1064 nm, i.e., in the range corresponding to the second transparency window of biological tissues. It can be seen (Fig. 5) that, for particles of both types, approximately the same inhibition of tumor growth is observed, while their doses differ nearly twofold. It should be noted that long-term (up to 9 months) observations of animals after intravenous administration of very large doses (45 mg of silver per 1 kg of weightFootnote 8) of a dispersion of composite particles with a silver shell have revealed no marked changes in their well-being or behavior. As a first approximation, this indicates a rather low toxicity of such particles.
Efficiency of PTT with the using composite particles containing iron oxyhydroxide cores and (1) gold or (2) silver shells, administered in a dose of 15 and 7 mg/kg, respectively. The insets show typical micrograph, extinction spectrum of particles, and the scheme of the experiment (based on the data of [101]).
In conclusion of this section, it should be noted that, at present, there is a clear trend towards a shift from basic researches to preclinical and clinical trials. (Therewith, the main attention is focused on the intravenous or intratumoral administration of the particles.) The first relatively successful example of this passage is AuroShell drug, which has been developed by AuroLase and is represented by a dispersion of spherical SiO2@Au core/shell composite particles. As has been evidenced by the results of the first stage of the clinical trials, this drug is quite efficient for the treatment of prostate cancer [99, 108]. Unfortunately, the process of the realization of thermoplasmonics in clinical practice is noticeably complicated by the lack of complete information about the systemic and chronic toxicity of NPs, as well as general ideas about their optimal structure, the most sensitive types of tumors, and the standards for PTT, including the method of the administration of the particles, irradiation conditions, etc. The most serious problems include the rapid accumulation of NPs in the liver and/or kidneys upon their intravenous administration. Another serious limitation is a rather high cost of gold-based drugs.
We believe that some of these problems can be solved, to a high extent, by using alternative methods of NP administration (primarily, transdermal and intranasal) and developing appropriate dosage forms. The promise of these approaches is, in particular, confirmed by the results of [109, 110]. For example, it has been shown that the use of polylactide microneedle patches coated with GNRs provides rather efficient PTT of human epidermal carcinoma [109].
4.2 Photothermal Theranostics of Cardiovascular Diseases
Cardiovascular diseases are the most common causes of death in the world, thus making it very important to develop methods for their diagnosis and treatment. Gold and silver NPs have begun to be used for this purpose relatively recently. Works [111, 112] are among the first studies in this area.
Work [111] was devoted to studying the possibility of using core/shell composite particles for the photothermal destruction of atherosclerotic plaques. Two approaches were compared. The first of them consisted in the application of a patch containing SiO2@Au core/shell particles onto an artery followed by irradiation of the plaque with a laser, which was introduced into the vessel and operated in the near-IR spectral region. Within the framework of the second approach, Fe3O4@SiO2@Au core/shell/shell particles were loaded into stem cells and, then, injected into the coronary artery through a catheter; an external laser was used for irradiation. It turned out that both approaches lead to a significant reduction in plaque volume [111]. This conclusion was also confirmed by clinical trials [113].
In turn, the authors of [112] showed the possibility of detecting atherosclerotic plaques by photoacoustic tomography on both phantoms and vessels ex vivo. In this case, GNRs with a silica shell were used as contrasting agents. The results of subsequent experiments in this area have indicated the promise of using plasmonic NPs of different sizes and shapes for photothermal theranostics of atherosclerosis and other diseases [114–118]. For example, according to the data of [115], composite particles with a magnetic iron oxide core and a silver shell provide not only efficient thrombus diagnostics in vivo, but also its photothermal lysis. In addition, it has been shown [118] that the introduction of GNRs into the left cervical ganglion of dogs followed by laser irradiation makes it possible to reversibly suppress the activity of neurons, thus reducing the likelihood of ventricular arrhythmia caused by myocardial infarction.
The results of the works cited above, first of all [113, 118], inspire restrained optimism about the potential of PTT as one of the methods for minimally invasive therapy of cardiovascular diseases.
4.3 The Use of Thermoplasmonics to Combat Bacterial and Viral Infections
The field of thermoplasmonics that implies the use of gold and silver NPs for the diagnosis and/or therapy of bacterial and viral infections can be attributed to one of the most promising fields both in terms of the achieved effects and the possibility of implementing results of laboratory studies in clinical practice.
A striking example of such implementation is the use of gold NPs and composite structures based thereon for creating photonic thermal cyclers, which can significantly accelerate the detection of infectious agents by means of polymerase chain reaction [7, 119–122]. The possibility of such acceleration was, for the first time, shown in 2015 using a thin gold film [119]. Currently, researchers actively develop miniature devices that enable one to point-of-care diagnostics of different diseases. The urgent need in such diagnostics has been revealed by the SARS-CoV-2 pandemic.
The ability of gold and silver NPs to photothermally suppress the vital activity of bacteria and viruses is of considerable interest for solving a wide spectrum of problems relevant to the sterilization of protective masks, medical instruments, and implants [123–125], the creation of new dressing materials (including those intended for treating infected wounds) [126–130], etc. [131–133]. Therewith, in the case of silver NPs, one can expect an additional effect caused by the action of silver ions (see, e.g., review [123] and references therein).
Experimental results [126–130] have indicated that the introduction of Au or Ag NPs into the matrix of a biocompatible polymer makes it possible to obtain dressings or templates for tissue engineering that can efficiently suppress infections (including those caused by bacteria resistant to antibiotics) both in vitro and in vivo. Irradiation with a laser operating in the transparency window of tissues promotes the photothermal death of bacteria, and, in some cases, enhances the proliferation of the cells healthy tissues, thereby accelerating wound healing.
Recently, works have begun to be published on the use of plasmonic NPs to combat infections of the respiratory tract or the central nervous system (CNS). For example, in the opinion of Prof. El-Sayed et al., bronchoscopic administration of GNRs that can specifically bind to the S-protein on the surface of SARS-CoV-2 (in combination with a subsequent PTT session) may become a new paradigm for the treatment of patients with severe lung damage caused by this virus [131].
In turn, the data of [132] have indicated that spherical gold NPs with a mixed monolayer of a quaternary ammonium compound and indocyanine green dye formed on their surface can serve as efficient means for combined therapyFootnote 9 of CNS injury by staphylococcus resistant to methicillin. In this case, laser irradiation not only promotes the photothermal death of bacteria, but also makes it possible to control the permeability of the blood-brain barrier.
In conclusion of this section, let us dwell on the prospects for the use of PTT in the treatment of acne. This seemingly non-serious disease can cause a number of psychological problems. The analysis of the literature [133–136] has indicated that PTT performed with the help of plasmonic NPs can lead both to the destruction Propionibacterium acnes, which are the causative agents of acne, and to photothermal ablation of the sebaceous glands. The highest efficiency of PTT has been observed with the use of a pulsed millisecond laser operating at 800 or 1064 nm and spherical composite particles composed of silica cores (d ≈ 120 nm) and gold shells nearly 15 nm thick [134, 136]. In the case of monotherapy, no acne recurrences are observed for at least three months, while the combination of PTT with a drug therapy aimed at regulating the intestinal microbiota can extend this period to two years [136]. In 2018, a drug based on SiO2/Au particles, which are produced by Sebacia Co. under trade name Sebacia microparticles, was subjected to clinical trials and approved by the United States Food and Drug Administration [137]. This method of acne treatment is most widely used in Asian countries.
4.4 The Use of Thermoplasmonics in Ophthalmology
The use of plasmonic NPs in ophthalmology makes it possible to solve a number of problems associated with the therapy and diagnosis of eye diseases (especially those occurring in the back of an eye). Such NPs can serve as contrasting agents in optical coherence or computer-assisted tomography, and also act as radiosensitizers, delivery vehicles for drugs and gene material, etc. [138].
The ability of NPs to transform the energy of absorbed radiation into heat is actively used to treat vitreous opacity [139] and postoperative complications in cataract or glaucoma [140–142], as well as to eliminate the dry eye syndrome [143].
For example, experiments in vitro and in vivo have shown that intravitreal administration of spherical gold NPs modified with hyaluronic acid and indocyanine green dye followed by pulsed laser irradiation promotes mechanical destruction of collagen clots that cause opacity of vitreous body [139]. This destruction occurs under the action of nanosized vapor bubbles resulting from the rapid evaporation of water near the NPs heated by laser pulses. An ideologically similar scheme was also employed for the treatment of fibrosis, which is one of the postoperative complications of glaucoma removal [142]. In this case, a peculiar composite based on silver NPs and graphene oxide was used as a thermosensitizer. According to the data obtained, PTT results in a significant decrease in the level of fibrosis and, as a consequence, a reduction in intraocular pressure.
The introduction of silica-coated GNRs or other gold-based structures into intraocular lenses causes the efficient thermal destruction of “residual” epithelial cells under the action of cw laser radiation, thereby preventing the cloudiness of the posterior lens capsule, which often occurs after cataract surgery [140, 141].
Summarizing all of the aforementioned in this section, it should be emphasized that, in our opinion, the results of the cited works have quite serious prospects to be implemented in clinical practice. This is, to a high extent, due to the possibility of the local administration of NPs, which makes it possible to minimize the risks associated with the accumulation of NPs in the body and their chronic toxicity.
4.5 The Use of Plasmonic NPs in the Treatment of Neurological Disorders and CNS Diseases
The central nervous system controls all vital functions of the body and communications with the environment. Any violation of its activity is associated with, at least, a significant decrease in the quality of life. As the analysis of the literature has shown [90, 144–152], the photothermal properties of plasmonic NPs determine their demand for minimally invasive diagnostics and/or treatment of a wide spectrum of CNS diseases.Footnote 10
In particular, nowadays a rapid increase is observed in the number of works devoted to the use of plasmonic NPs for modulating the activity of neurons and regulating the differentiation stem cells [144–152]. As a first approximation, they may be divided into two groups. One of them includes studies aimed at the determination of the optimal conditions for laser irradiation to prevent cells from death as a result of thermal shock (see, e.g., [147] and references therein). Another group comprises works devoted to determining the efficiency of this approach within various models using both dispersions of NPs and their ensembles formed on various substrates and developing methods for creating neuroprostheses [144–146, 148–152]Footnote 11.
In the opinion of most authors (see reviews [144–146]), photomodulation of neuron activity mediated by plasmonic NPs improves not only visual and auditory, but also cognitive functions. (The latter is especially important as applied to the treatment of the Alzheimer, Parkinson, and other neurodegenerative disorders.) For example, it has been shown that GNRs can be used both to stimulate and to inhibit the activity of retinal ganglion cells [150] under the action of pulsed laser radiation. At the same time, short laser pulses promoted an increase in cell activity, while long pulses suppressed it. The obtained information can be used as the basis for the creation of ocular neuroprostheses.
The mechanisms of neuron photomodulation in the presence of plasmonic NPs still remain to be studied; however, it is assumed that they are based on the optical stimulation of thermosensitive ion channels (in particular, the TREK-1 potassium channel) [144].
In addition to studying the mechanisms that govern the behavior of nerve cells, further researches are needed to determine the biocompatibility and efficiency of the devices being developed. Nevertheless, taking into account the rapid development of this field of knowledge, it can be assumed that clinical trials of new types of neuroprostheses will begin in the nearest future.
4.6 Plasmonic NPs as Means for Intracellular Delivery of Biologically Active Compounds
An increase in the permeability of a cell membrane due to laser irradiation or optoporation is one of the promising methods for both transfection of cells (i.e., the introduction of foreign nucleic acids) and the delivery of various compounds (e.g., drugs) [7, 154, 155].
The use of plasmonic NPs makes it possible to substantially increase the efficiency of this process. Lapotko et al. were the first to show the possibility of the transfection by the example of spherical gold NPs and a number of cell cultures [156]. As the experimental results have shown, NPs penetrating into cells are localized near the membrane to form small aggregates. Irradiation of such a system with a nanosecond laser leads to the instantaneous evaporation of water near the NP surface and the formation of nanosized vapor bubbles. In the course of their growth and collapse, the membrane is deformed with the formation of peculiar channels in it, which let plasmid DNA penetrate into the cell [156].
In the course of subsequent studies of the features of optoporation and transfection of cells in suspensions and on substrates,Footnote 12 the optimal parameters of laser irradiation, the rate of the restoration of the integrity of the cell membrane, and other factors controlling the course of these processes were determined [154, 155, 157–160]. It has, in particular, been shown that two-dimensional ensembles of gold nanostars formed on a silicon substrate modified with poly(vinylpyridine) could serve as a “platform” for transfection of HeLa cells with plasmid DNA, with this process proceeding under the action of a cw laser radiation at a wavelength of 808 nm [157]. The transfection efficiency quite expectedly depended on the density of the nanoparticle ensemble and the laser radiation energy. Moreover, the “working” range was determined for heating of the cell suspension under laser irradiation (approximately, 42−53°C) corresponding to the combination of high levels of transfection and cell survival.
The feasibility of using cw lasers for optoporation increases the availability of this approach taking into account their relatively low cost compared to pulsed lasers. However, it should be kept in mind that their use may be more traumatic for cells. This is indirectly indicated by the results of [158]. For example, the complete restoration of the integrity of a cell membrane after the cw laser irradiation takes much longer time than after the pulsed irradiation.
Although the data of the cited works indicate the promise of using plasmonic NPs for the intracellular delivery of gene materials and various drugs, this approach is difficult to implement in the clinical practice. One of the main reasons for this situation is the lack of sufficiently complete information about the mechanisms of the processes underlying plasmon-induced optoporation.
5 CONCLUSIONS
The unique properties of gold and silver NPs determine their surprisingly happy “scientific fortune,” reflected in the continuously growing interest of researchers in the problems relevant to the deepening of ideas about the features of the interaction of such NPs with radiation in different frequency ranges, the development of new methods for regulating their morphology, as well as their use for creating new materials and devices for various purposes.
This review, which has shown a wide spectrum of possibilities that open up in biomedicine owing to the ability of gold and silver NPs to transform the energy of absorbed radiation into heat, is a quite convincing confirmation of the aforementioned.
The analysis of the literature data has indicated that, in addition to a significant increase in the number of articles devoted to the development of new approaches to the use of plasmonic NPs and the determination of the mechanisms underlying this or that process, there is a clear trend towards a gradual transition from laboratory studies to preclinical trials, and, in some cases, and to clinical practice.
A particularly urgent task is, undoubtedly, the implementation of new methods for the treatment of oncological diseases in the practical medicine and the creation of appropriate dosage forms based on plasmonic NPs. As has been noted above, one of the main obstacles on this way is the ability of such particles to be actively accumulated in the liver and some other organs. To a high extent, the local introduction of NPs makes it possible to cope with this problem. In addition, some optimism is inspired by the results of recent study [161], which has proposed a method for the temporary partial “blockage” of mononuclear phagocytes responsible for removing foreign particles from the bloodstream.Footnote 13 This makes it possible to significantly increase the duration of circulating target particles and, consequently, the likelihood of their accumulation in a tumor.
It should be emphasized that the use of plasmonic NPs in PTT is not confined to the above areas. Indeed, recent works have indicated the possibility of efficient use of PTT in dentistry [162], treatment of liver fibrosis [163], rheumatoid arthritis [164], venous malformation [165], as well as in stimulating osteogenesis [166]. Moreover, it is obvious that PTT can be combined with other types of therapy in the treatment of not only tumors, but also other diseases.
Notes
This tendency is typical for particles of all types.
The concentrations of Au and Ag in the studied dispersions were ≈0.4 and ≈0.15 mM, respectively. As a consequence, the difference between the PTEs of particles of two types per unit mass of metal will be even more significant.
The LSPR of the particles coincided with the laser generation wavelength and was in the near-IR range, which corresponded to the highest transmission (“transparency window”) of biological tissues.
Review [90] is devoted to the detailed description of the potentials and advantages of multimodal therapy.
Strictly speaking, all these particles (with the exception of the core/shell structures) rather consist of gold-silver alloys, since one of the components used in their synthesis is silver nitrate.
It should be emphasized that there are actually no works devoted to systematic comparison between the toxicities of NPs of these two metals (especially with regard to their sizes and surface chemistry).
More than six times higher than the therapeutic dose used in the experiments in [101].
We mean the combination of PTT with medicinal and photodynamic effects.
In this case, we are talking only about gold NPs. On the contrary, silver NPs are actually not used in such experiments because of their rather high neurotoxicity [153].
Note that, in this case, only gold NPs were used in the experiments.
It is based on the intravenous administration of allogeneic anti-erythrocyte antibodies [162].
REFERENCES
Kreibig, U. and Volmer, M., Optical Properties of Metal Clusters, Berlin: Springer-Verlag, 1995.
Klimov, V.V., Nanoplazmonika (Nanoplasmonics), Moscow: Fizmatlit, 2009.
Active Plasmonics and Tuneable Plasmonic Metamaterials, Zayats, A.V. and Maier, S., Eds., Hoboken: J. Wiley & Sons Inc. and ScienceWise Publishing, 2013.
Baev, A., Prasad, P.N., Egren, H., Samoć, M., and Wegener, M, Metaphotonics: An emerging field with opportunities and challenges, Phys. Rep., 2015, vol. 594, p. 1.
Coronado, E.A., Encina, E.R., and Stefani, F.D., Optical properties of metallic nanoparticles: Manipulating light, heat and forces at the nanoscale, Nanoscale, 2011, vol. 3, p. 4042.
Photothermal Nanomaterials, Ye, E. and Li, Z., Eds., London: The Royal Society of Chemistry, 2022.
Jauffred, L., Samadi, A., Klingberg, H., Bendix, P.M., and Oddershede, L.B., Plasmonic heating of nanostructures, Chem. Rev., 2019, vol. 119, p. 8087.
Baffou, G., Cichos, F., and Quidant, R., Applications and challenges of thermoplasmonics, Nat. Mater., 2020, vol. 19, p. 946.
Guglielmelli, A., Pierini, F., Tabiryan, N., Umeton, C., Bunning, T.J., and De Sio, L., Thermoplasmonics with gold nanoparticles: A new weapon in modern optics and biomedicine, Adv. Photonics Res., 2021, vol. 2, p. 2000198.
Webb, J.A. and Bardhan, R., Emerging advances in nanomedicine with engineered gold nanostructures, Nanoscale, 2014, vol. 6, p. 2502.
Hashimoto, S., Werner, D., and Uwada, T., Studies on the interaction of pulsed lasers with plasmonic gold nanoparticles toward light manipulation, heat management, and nanofabrication, J. Photochem. Photobiol., C, 2012, vol. 13, p. 28.
Baffou, G. and Rigneault, H., Femtosecond-pulsed optical heating of gold nanoparticles, Phys. Rev. B, 2011, vol. 84, p. 035415.
Ekici, O., Harrison, R.K., Durr, N.J., Eversole, D.S., Lee, M., and Ben-Yakar, A., Thermal analysis of gold nanorods heated with femtosecond laser pulses, J. Phys. D: Appl. Phys., 2008, vol. 41, p. 185501.
Huang, W., Qian, W., El-Sayed, M.A., Ding, Y., and Wang, Z.L., Effect of the lattice crystallinity on the electron−phonon relaxation rates in gold nanoparticles, J. Phys. Chem. C, 2007, vol. 111, p. 10751.
Pustovalov, V. and Zharov, V., Threshold parameters of the mechanisms of selective nanophotothermolysis with gold nanoparticles, Proc. Int. Soc. Opt. Eng., 2008, vol. 6854, p. 685412-1.
Huang, X. and El-Sayed, M.A., Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy, J. Adv. Res., 2010, vol. 1, p. 13.
Werner, D., Furube, A., Okamoto, T., and Hashimoto, S., Femtosecond laser-induced size reduction of aqueous gold nanoparticles: in situ and pump−probe spectroscopy investigations revealing Coulomb explosion, J. Phys. Chem. C, 2011, vol. 115, p. 8503.
Qin, Z. and Bischof, J.C., Thermophysical and biological responses of gold nanoparticle laser heating, Chem. Soc. Rev., 2012, vol. 41, p. 1191.
Khlebtsov, N.G. and Dykman, L.A., Optical properties and biomedical applications of plasmonic nanoparticles, J. Quant. Spectrosc. Radiat. Transfer, 2010, vol. 111, p. 1.
Boulais, E., Lachaine, R., Hatef, A., and Meunier, M., Plasmonics for pulsed-laser cell nanosurgery: Fundamentals and applications, J. Photochem. Photobiol., C, 2013, vol. 17, p. 26.
Jiang, R., Cheng, S., Shao, L., Ruan, Q., and Wang, J., Mass-based photothermal comparison among gold nanocrystals, PbS nanocrystals, organic dyes, and carbon black, J. Phys. Chem. C, 2013, vol. 117, p. 8909.
Lalisse, A., Tessier, G., Plain, J., and Baffou, G., Quantifying the efficiency of plasmonic materials for near-field enhancement and photothermal conversion, J. Phys. Chem. C, 2015, vol. 119, p. 25518.
Gutiérrez, Y., Losurdo, M., González, F., Everitt, H.O., and Moreno, F., Nanoplasmonic photothermal heating and near-field enhancements: A comparative survey of 19 metals, J. Phys. Chem. C, 2020, vol. 124, p. 7386.
Pathak, N.K., Sarathi, P., and Pandey, G.K., Comparative study of thermoplasmonic effects of gold and silver metal nanoparticle, AIP Adv., 2021, vol. 11, p. 045323.
Pásciak, A., Marin, R., Abiven, L., Pilch-Wrobel, A., Misiak, M., Xu, W., Prorok, K., Bezkrovnyi, O., Marciniak, Ł., Chaneac, C., Gazeau, F., Bazzi, R., Roux, S., Viana, B., Lehto, V.-P., Jaque, D., and Bednarkiewicz, A., Quantitative comparison of the light-to-heat conversion efficiency in nanomaterials suitable for photothermal therapy, ACS Appl. Mater. Interfaces, 2022, vol. 14, p. 33555.
Bastús, N.G., Comenge, J., and Puntes, V., Kinetically controlled seeded growth synthesis of citrate-stabilized gold nanoparticles of up to 200 nm: Size focusing versus ostwald ripening, Langmuir, 2011, vol. 27, p. 11098.
Bastús, N.G., Merkoçi, F., Piella, J., and Puntes, V., Synthesis of highly monodisperse citrate-stabilized silver nanoparticles of up to 200 nm: Kinetic control and catalytic properties, Chem. Mater., 2014, vol. 26, p. 2836.
Pelaz, B., Grazu, V., Ibarra, A., Magen, C., del Pino, P., and de la Fuente, J.M., Tailoring the synthesis and heating ability of gold nanoprisms for bioapplications, Langmuir, 2012, vol. 28, p. 8965.
Zhu, J., Yong, K.-T., Roy, I., Hu, R., Ding, H., Zhao, L., Swihart, M.T., He, G.S., Cui, Y., and Prasad, P.N., Additive controlled synthesis of gold nanorods (GNRs) for two-photon luminescence imaging of cancer cells, Nanotecnology, 2010, vol. 21, p. 285106.
Salavatov, N.A., Dement’eva, O.V, Mi-khailichenko, A.l., and Rudoy, V.M., Some aspects of seedless synthesis of gold nanorods, Colloid J., 2018, vol. 80, p. 541.
Wang, H., Brandl, D.W., Le, F., Nordlander, P., Halas, N.J., Nanorice: A hybrid plasmonic nanostructure, Nano Lett., 2006, vol. 6, p. 827.
Loo, C., Lin, A., Hirsch, L., Lee, M.-H., Barton, J., Halas, N., West, J., and Drezek, R., Nanoshell-enabled photonics-based imaging and therapy of cancer, Technol. Cancer Res. Treat., 2004, vol. 3, p. 33.
Skrabalak, S.E., Au, L., Li, X., and Xia, Y., Facile synthesis of Ag nanocubes and Au nanocages, Nat. Protoc., 2007, vol. 2, p. 2182.
Mulvaney, P., Giersig, M., and Henglein, A., Electrochemistry of multilayer colloids: Preparation and absorption spectrum of gold-coated silver particles, J. Phys. Chem., 1993, vol. 97, p. 7061.
Wang, H., Brandl, D.W., Nordlander, P., and Halas, N., Plasmonic nanostructures: Artificial molecules, Acc. Chem. Res., 2007, vol. 40, p. 53.
Lal, S., Link, S., and Halas, N.J., Nano-optics from sensing to waveguiding, Nat. Photonics, 2007, vol. 1, p. 641.
Omrani, M., Mohammadi, H., and Fallah, H., Ultrahigh sensitive refractive index nanosensors based on nanoshells, nanocages and nanoframes: Effects of plasmon hybridization and restoring force, Sci. Rep., 2021, vol. 11, p. 2065.
Erickson, T.A. and Tunnell, J.W., Nanomaterials for the Life Sciences, vol. 3: Mixed Metal Nanomaterials, Kumar, C.S.S.R., Ed., Weinheim: Wiley-VCH, 2009, p. 1.
Jain, P.K., Lee, K.S., El-Sayed, I.H., and El-Sayed, M.A., Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine, J. Phys. Chem. B, 2006, vol. 110, p. 7238.
Park, K., Biswas, S., Kanel, S., Nepal, D., and Vaia, R.A., Engineering the optical properties of gold nanorods: Independent tuning of surface plasmon energy, extinction coefficient, and scattering cross section, J. Phys. Chem. C, 2014, vol. 118, p. 5918.
Westcott, S.L., Jackson, J.B., Radloff, C., and Halas, N.J., Relative contributions to the plasmon line shape of metal nanoshells, Phys. Rev. B, 2002, vol. 66, p. 155431.
Khlebtsov, N.G., Dykman, L.A., and Khlebtsov, B.N., Synthesis and plasmonic tuning of gold and gold–silver nanoparticles, Russ. Chem. Rev., 2022, vol. 91, p. RCR5058.
Barbosa, S., Agrawal, A., Rodríguez-Lorenzo, L., Pastoriza-Santos, I., Alvarez-Puebla, A.R., Kornow-ski, A., Weller, H., and Liz-Marzán, L.M., Tuning size and sensing properties in colloidal gold nanostars, Langmuir, 2010, vol. 26, p. 14943.
Scarabelli, L., Coronado-Puchau, M., Giner-Casares, J.J., Langer, J., and Liz-Marzan, L.M., Monodisperse gold nanotriangles: Size control, large-scale self-assembly, and performance in surface-enhanced raman scattering, ACS Nano, 2014, vol. 8, p. 5833.
Zheng, J., Cheng, X., Zhang, H., Bai, X., Ai, R., Shao, L., and Wang, J., Gold nanorods: The most versatile plasmonic nanoparticles, Chem. Rev., 2021, vol. 121, p. 13342.
Gole, A. and Murphy, C.J., Seed-mediated synthesis of gold nanorods: Role of the size and nature of the seed, Chem. Mater., 2004, vol. 16, p. 3633.
Siegel, A.L. and Baker G.A., Bespoke nanostars: Synthetic strategies, tactics, and uses of tailored branched gold nanoparticles, Nanoscale Adv., 2021, vol. 3, p. 3980.
Roach, L., Coletta, P.L., Critchley, K., and Evans, S.D., Controlling the optical properties of gold nanorods in one-pot syntheses, J. Phys. Chem. C, 2022, vol. 126, p. 3235.
Skrabalak, S.E., Chen, J., Sun, Y., Lu, X., Au, L., Cobley, C.M., and Xia, Y., Gold nanocages: Synthesis, properties, and applications, Acc. Chem. Res., 2008, vol. 41, p. 1587.
Adams, S. and Zhang, J.Z., Unique optical properties and applications of hollow gold nanospheres (HGNs), Coord. Chem. Rev., 2016, vols. 320−321, p. 18.
Aherne, D., Gara, M., Kelly, J.M., and Gun’ko, Y.K., From Ag nanoprisms to triangular AuAg nanoboxes, Adv. Funct. Mater., 2010, vol. 20, p. 1329.
Maksimova, E.A., Barmin, R.A., Rudakovskaya, P.G., Sindeeva, O.A., Prikhozhdenko, E.S., Yash-chenok, A.M., Khlebtsov, B.N., Solovev, A.A., Huang, G., Mei, Y., Dey, K.K., and Gorin, D.A., Air-filled microbubbles based on albumin functionalized with gold nanocages and zinc phthalocyanine for multimodal imaging, Micromachines, 2021, vol. 12, p. 1161.
Dement’eva, O.V. and Rudoy, V.M., Colloidal synthesis of new silver-based nanostructures with tailored localized surface plasmon resonance, Colloid J., 2011, vol. 73, p.724.
Rycenga, M., Cobley, C.M., Zeng, J., Li, W., Moran, C.H., Zhang, Q., Qin, D., and Xia, Y., Controlling the synthesis and assembly of silver nanostructures for plasmonic applications, Chem. Rev., 2011, vol. 111, p. 3669.
Kartseva, M.E., Dement’eva, O.V., Filippenko, M.A., and Rudoy, V.M., Anisotropic particles with different morphologies of silver nanoshell: Synthesis and optical properties, Colloid J., 2011, vol. 73, p. 340.
Panfilova, E.V., Khlebtsov, B.N., Burov, A.M., and Khlebtsov, N.G., Study of polyol synthesis reaction parameters controlling high yield of silver nanocubes, Colloid J., 2012, vol. 74, p. 99.
Nasilowski, M., Mahler, B., Lhuillier, E., Ithurria, S., and Dubertret, B., Two-dimensional colloidal nanocrystals, Chem. Rev., 2016, vol. 116, p. 10934.
Khodashenas, B. and Ghorbani, H.R., Synthesis of silver nanoparticles with different shapes, Arabian J. Chem., 2019, vol. 12, p. 1823.
Shi, Y., Lyu, Z., Zhao, M., Chen, R., Nguyen, Q.N., and Xia, Y., Noble-metal nanocrystals with controlled shapes for catalytic and electrocatalytic applications, Chem. Rev., 2021, vol. 121, p. 649.
Khlebtsov, B.N., Khanadeev, V.A., Maksimova, I.L., Terentyuk, G.S., and Khlebtsov, N.G., Silver nanocubes and gold nanocages: Their synthesis and optical and photothermal properties, Nanotechnol. Russ., 2010, vol. 5, p. 454.
Chen, H., Shao, L., Ming, T., Sun, Z., Zhao, C., Yang, B., and Wang, J., Understanding the photothermal conversion efficiency of gold nanocrystals, Small, 2010, vol. 6, p. 2272.
Cole, J.R., Mirin, N.A., Knight, M.W., Goodrich, G.P., and Halas, N.J., Photothermal efficiencies of nanoshells and nanorods for clinical therapeutic applications, J. Phys. Chem. C, 2009, vol. 113, p. 12090.
Zeng, J., Goldfeld, D., and Xia, Y., A plasmon-assisted optofluidic (PAOF) system for measuring the photothermal conversion efficiencies of gold nanostructures and controlling an electrical switch, Angew. Chem., Int. Ed. Engl., 2013, vol. 52, p. 4169.
Espinosa, A., Kolosnjaj-Tabi, J., Abou-Hassan, A., Sangnier, A.P., Curcio, A., Silva, A.K.A., Di Corato, R., Neveu, S., Pellegrino, T., Liz-Marzán, L.M., and Wilhelm, C., Magnetic (hyper)thermia or photothermia? Progressive comparison of iron oxide and gold nanoparticles heating in water, in cells, and in vivo, Adv. Funct. Mater., 2018, vol. 28, p. 1803660.
Huang, P., Rong, P., Lin, J., Li, W., Yan, X., Zhang, M.G., Nie, L., Niu, G., Lu, J., Wang, W., and Chen, X., Triphase interface synthesis of plasmonic gold bellflowers as near-infrared light mediated acoustic and thermal theranostics, J. Am. Chem. Soc., 2014, vol. 136, p. 8307.
Ayala-Orozco, C., Urban, C., Knight, M.W., Urban, A.S., Neumann, O., Bishnoi, S.W., Mukherjee, S., Goodman, A.M., Charron, H., Mitchell, T., Shea, M., Roy, R., Nanda, S., Schiff, R., Halas, N.J., and Joshi, A., Au nanomatryoshkas as efficient near-infrared photothermal transducers for cancer treatment: Benchmarking against nanoshells, ACS Nano, 2014, vol. 8, p. 6372.
Pattani, V.P. and Tunnell, J.W., Nanoparticle-mediated photothermal therapy: A comparative study of heating for different particle types, Lasers Surg. Med., 2012, vol. 44, p. 675.
Liu, P., Wang, Y., Liu, Y., Tan, F., Li, J., and Li, N., S-nitrosothiols loaded mini-sized Au@silica nanorod elicits collagen depletion and mitochondrial damage in solid tumor treatment, Theranostics, 2020, vol. 10, p. 6774.
Mondal, S., Montaño-Priede, J.L., Nguyen, V.T., Park, S., Choi, J., Doan, V.H.M., Vo, T.M.T., Vo, T.H., Large, N., Kim, C.-S., and Oh, J., Computational analysis of drug free silver triangular nanoprism theranostic probe plasmonic behavior for in-situ tumor imaging and photothermal therapy, J. Adv. Res., 2022, vol. 41, p. 23.
Chen, Y.-S., Frey, W., Kim, S., Homan, K., Kruizinga, P., Sokolov, K., and Emelianov, S., Enhanced thermal stability of silica-coated gold nanorods for photoacoustic imaging and image-guided therapy, Opt. Express, 2010, vol. 18, p. 8867.
Khanadeev, V.A., Simonenko, A.V., Grishin, O.V., and Khlebtsov, N.G., One-shot laser-pulse modification of bare and silica-coated gold nanoparticles of various morphologies, Nanomaterials, 2023, vol. 13, p. 1312.
Croissant, J.G. and Guardado-Alvarez, T.M., Photocracking silica: Tuning the plasmonic photothermal degradation of mesoporous silica encapsulating gold nanoparticles for cargo release, Inorganics, 2019, vol. 7, p. 72.
Gonçalves, A.S.C., Rodrigues, C.F., Moreira, A.F., and Correia, I.J., Strategies to improve the photothermal capacity of gold-based nanomedicines, Acta Biomater., 2020, vol. 116, p. 105.
Salavatov, N.A., Bol’shakova, A.V., Morozov, V.N., Kolyvanova, M.A., Isagulieva, A.K., and Dement’eva, O.V., Gold nanorods with functionalized organosilica shells: Synthesis and prospects of application in tumor theranostics, Colloid J., 2022, vol. 84, p. 93.
Qiu, J. and Wei, W.D., Surface plasmon-mediated photothermal chemistry, J. Phys. Chem. C, 2014, vol. 118, p. 20735.
Pandres, E.P., Crane, M.J., Davis, E.J., Pauzauskie, P.J., and Holmberg, V.C., Laser-driven growth of semiconductor nanowires from colloidal nanocrystals, ACS Nano, 2021, vol. 15, p. 8653.
Song, C., Wang, Z., Yin, Z., Xiao, D., and Ma, D., Principles and applications of photothermal catalysis, Chem. Catal., 2022, vol. 2, p. 52.
Ye, H., Li, X., Deng, L., Li, P., Zhang, T., Wang, X., and Hsiao, B.S., Silver nanoparticle-enabled photothermal nanofibrous membrane for light-driven membrane distillation, Ind. Eng. Chem. Res., 2019, vol. 58, p. 3269.
Alessandro, F., Macedonio, F., and Drioli, E., Plasmonic phenomena in membrane distillation, Membranes, 2023, vol. 13, p. 254.
Shi, Y., Zhang, C., Wang, Y., Cui, Y., Wang, Q., Liu, G., Gao, S., and Yuan, Y., Plasmonic silver nanoparticles embedded in flexible three-dimensional carbonized melamine foam with enhanced solar-driven water evaporation, Desalination, 2021, vol. 507, p. 115038.
Sharma, P., Daipuriya, R., and Singh, M., Growth of V2O5 nanopillers using plasma assisted oxidation cum sublimation, AIP Conf. Proc., 2020, vol. 2220, p. 020035.
Fang, X., Sharma, M., Stennett, C., and Gill, P.P., Optical sensitisation of energetic crystals with gold nanoparticles for laser ignition, Combust. Flame, 2017, vol. 183, p. 15.
Fang, X., Stone, M., and Stennett, C., Pulsed laser irradiation of a nanoparticles sensitised RDX crystal, Combust. Flame, 2020, vol. 214, p. 387.
Churchyard, S., Fang, X., and Vrcelj, R., Laser ignitibility of energetic crystals doped with gold nanoparticles, Opt. Laser Technol., 2019, vol. 113, p. 281.
Dai, Y., He, G., Long, S., Li, X., Meng, L., Wang, P., Li, X., and Yang, Z., Precise tailoring of mesoporous silica-coated gold nanorods for laser ignition at 1064 nm, ACS Appl. Nano Mater., 2023, vol. 6, p. 4946.
Hirsch, L.R., Stafford, R.J., Bankson, J.A., Sershen, S.R., Rivera, B., Price, R.E., Hazle, J.D., Halas, N.J., and West, J.L., Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance, Proc. Natl. Acad. Sci. U.S.A., 2003, vol. 100, p. 13549.
Yang, X., Yang, M., Pang, B., Vara, M., and Xia, Y., Gold nanomaterials at work in biomedicine, Chem. Rev., 2015, vol. 115, p. 10410.
Izci, M., Maksoudian, C., Manshian, B.B., and Soenen, S.J., The use of alternative strategies for enhanced nanoparticle delivery to solid tumors, Chem. Rev., 2021, vol. 121, p. 1746.
Kang, S., Bhang, S.H., Hwang, S., Yoon, J.K., Song, J., Jang, H.K., Kim, S., and Kim, B.S., Mesenchymal stem cells aggregate and deliver gold nanoparticles to tumors for photothermal therapy, ACS Nano, 2015, vol. 9, p. 9678.
Fan, W., Yung, B., Huang, P., and Chen, X., Nanotechnology for multimodal synergistic cancer therapy, Chem. Rev., 2017, vol. 117, p. 13566.
Ali, M.R.K., Wu, Y., and El-Sayed, M.A., Gold-nanoparticle-assisted plasmonic photothermal therapy advances toward clinical application, J. Phys. Chem. C, 2019, vol. 123, p. 15375.
Ali, M.R., Ibrahim, I.M., Ali, H.R., Selim, S.A., and El-Sayed, M., Treatment of natural mammary gland tumors in canines and felines using gold nanorods-assisted plasmonic photothermal therapy to induce tumor apoptosis, Int. J. Nanomed., 2016, vol. 11, p. 4849.
Ali, M.R.K., Wu, Y., Tang, Y., Xiao, H., Chen, K., Han, T., Fang, N., Wu, R., and El-Sayed, M.A., Targeting cancer cell integrins using gold nanorods in photothermal therapy inhibits migration through affecting cytoskeletal proteins, Proc. Natl. Acad. Sci. U.S.A., 2017, vol. 114, p. e5655.
Liu, Y., Chongsathidkiet, P., Crawford, B.M., Odion, R., Dechant, C.A., Kemeny, H.R., Cui, X., Maccarini, P.F., Lascola, C.D., Fecci, P.E., and Vo-Dinh, T., Plasmonic gold nanostar-mediated photothermal immunotherapy for brain tumor ablation and immunologic memory, Immunotherapy, 2019, vol. 11, p. 1293.
Abadeer, N.S. and Murphy, C.J., Recent progress in cancer thermal therapy using gold nanoparticles, J. Phys. Chem. C, 2016, vol. 120, p. 4691.
Kim, M., Lee, J.-H., and Nam, J.-M., Plasmonic photothermal nanoparticles for biomedical applications, Adv. Sci., 2019, vol. 6, p. 1900471.
Zhou, R., Zhang, M., Xi, J., Li, J., Ma, R., Ren, L., Bai, Z., Qi, K., and Li, X., Gold nanorods-based photothermal therapy: Interactions between biostructure, nanomaterial, and near-infrared irradiation, Nanoscale Res. Lett., 2022, vol. 17, p. 68.
Piao, J.-G., Liu, D., Hu, K., Wang, L., Gao, F., Xiong, Y., and Yang, L., Cooperative nanoparticle system for photothermal tumor treatment without skin damage, ACS Appl. Mater. Interfaces, 2016, vol. 8, p. 2847.
Yang, Y., Zheng, X., Chen, L., Gong, X., Yang, H., and Duan, X.ZhuY., Multifunctional gold nanoparticles in cancer diagnosis and treatment, Int. J. Nanomed., 2022, vol. 17, p. 2041.
Yakubovskaya, R.I., Pankratov, A.A., Andreeva, T.N., Venediktova, Yu.B., Kogan, B.Ya., Butenin, A.V., Puchnova, V.A., Fezulova, R.A., Rudoi, V.M., Dement’eva, O.V., Kartseva, M.E., Filippenko, M.A., Chissov, V.I., and Vorozhtsov, G.N., Pulsed laser hyperthermia with nanoparticles as thermosensitizers is a new potential method of anticancer therapy, Rossiiskii Onkologicheskii Zhurnal, 2010, no. 6, p. 32.
Dement’eva, O.V., Filippenko, M.A., Kartseva, M.E., Sedykh, E.M., Bannykh, L.N., Yakubovskaya, R.I., Pankratov, A.A., Kogan, B.Ya., and Rudoy, V.M., Synthesis of anisotropic plasmonic nanoparticles with core-shell structure and prospects of their application in laser treatment of tumors, Nanotechnol. Russ., 2012, vol. 7, nos. 9−10, p. 517.
Sears, J., Swanner, J., Fahrenholtz, C.D., Snyder, C., Rohde, M., Levi-Polyachenko, N., and Singh, R., Combined photothermal and ionizing radiation sensitization of triple-negative breast cancer using triangular silver nanoparticles, Int. J. Nanomed., 2021, vol. 16, p. 851.
Graham, E., Macneill C.M., Young M., Donati G., Wailes E.M., Jones B.T., and Levi-Polyachen-ko N.H., Differential response of MCF7, MDA-MB-231, and MCF 10A cells to hyperthermia, silver nanoparticles and silver nanoparticle-induced photothermal therapy, Int. J. Hyperthermia, 2014, vol. 30, p. 312.
Patlolla, A.K., Hackett, D., and Tchounwou, P.B., Silver nanoparticle-induced oxidative stress-dependent toxicity in Sprague−Dawley rats, Mol. Cell. Biochem., 2015, vol. 399, p. 257.
Guo, D., Zhu, L., Huang, Z., Zhou, H., Ge, Y., Ma, W., Wu, J., Zhang, X., Zhou, X., Zhang, Y., Zhao, Y., and Gu, N., Anti-leukemia activity of PVP-coated silver nanoparticles via generation of reactive oxygen species and release of silver ions, Biomaterials, 2013, vol. 34, p. 7884.
Tambunlertchai, S., Geary, S.M., Naguib, Y.W., and Salem, A.K., Investigating silver nanoparticles and resiquimod as a local melanoma treatment, Eur. J. Pharm. Biopharm., 2023, vol. 183, p. 1.
Swanner, J., Mims, J., Carroll, D.L., Akman, S.A., Furdui, C.M., Torti, S.V., and Singh, R.N., Differential cytotoxic and radiosensitizing effects of silver nanoparticles on triple-negative breast cancer and non-triple-negative breast cells, Int. J. Nanomed., 2015, vol. 10, p. 3937.
Zhang R., Kiessling F., Lammers T., Pallares R.M. Clinical translation of gold nanoparticles Drug Deliv. and Transl. Res., 2023, vol. 13, p. 378.
Hao, Y., Dong, M.L., Zhang, T.Y., Peng, J.R., Jia, Y.P., Cao, Y.P., and Qian, Z.Y., Novel approach of using near-infrared responsive PEGylated gold nanorod coated poly(l-lactide) microneedles to enhance the antitumor efficiency of docetaxel-loaded MPEG-PDLLA micelles for treating an A431 tumor, ACS A-ppl. Mater. Interfaces, 2017, vol. 9, p. 15317.
Wang, L., Tang, S., Yu, Y., Lv, Y., Wang, A., Yan, X., Li, N., Sha, C., Sun, K., and Li, Y., Intranasal delivery of temozolomide-conjugated gold nanoparticles functionalized with Anti-EphA3 for glioblastoma targeting, Mol. Pharmaceutics, 2021, vol. 18, p. 915.
Kharlamov, A.N. and Gabinsky, J.L., Plasmonic photothermic and stem cell therapy of atherosclerotic plaque as a novel nanotool for angioplasty and artery remodeling, Rejuvenation Res., 2012, vol. 15, p. 222.
Yeager, D., Chen, Y.-S., Litovsky, S., and Emelianov, S., Intravascular photoacoustics for image-guidance and temperature monitoring during plasmonic photothermal therapy of atherosclerotic plaques: A feasibility study, Theranostics, 2014, vol. 4, p. 36.
Kharlamov, A.N., Tyurnina, A.E., Veselova, V.S., Kovtun, O.P., Shur, V.Y., and Gabinsky, J.L., Silica-gold nanoparticles for atheroprotective management of plaques: Results of the NANOM-FIM trial, Nanoscale, 2015, vol. 7, p. 8003.
Qin, J., Peng, Z., Li, B., Ye, K., Zhang, Y., Yuan, F., Yang, X., Huang, L., Hu, J., and Lu, X., Gold nanorods as a theranostic platform for in vitro and in vivo imaging and photothermal therapy of inflammatory macrophages, Nanoscale, 2015, vol. 7, p. 13991.
Vazquez-Prada, K.X., Moonshi, S.S., Wu, Y., Akther, F., Tse, B.W.C., Sokolowski, K.A., Peter, K., Wang, X., Xu, G., and Ta, H.T., A spiky silver-iron oxide nanoparticle for highly efficient targeted photothermal therapy and multimodal imaging of thrombosis, Small, 2023, vol. 19, p. 2205744.
Dai, T., He, W., Yao, C., Ma, X., Ren, W., Mai, Y., and Wu, A., Applications of inorganic nanoparticles in the diagnosis and therapy of atherosclerosis, Biomater. Sci., 2020, vol. 8, p. 3784.
Hu, Q., Fang, Z., Ge, J., and Li, H., Nanotechnology for cardiovascular diseases, Innovation, 2022, vol. 3, p. 100214.
Ye, T., Lai, Y., Wang, Z., Zhang, X., Meng, G., Zhou, L., Zhang, Y., Zhou, Z., Deng, J., Wang, M., Wang, Y., Zhang, Q., Zhou, X., Yu, L., Jiang, H., and Xiao, X., Precise modulation of gold nanorods for protecting against malignant ventricular arrhythmias via near-infrared neuromodulation, Adv. Funct. Mater., 2019, vol. 29, p. 1902128.
Son, J.H., Cho, B., Hong, S., Lee, S.H., Hoxha, O., Haack, A.J., and Lee, L.P., Ultrafast photonic PCR, Light: Sci. Appl., 2015, vol. 4, p. e280.
Jiang, K., Wu, J., Qiu, Y., Go, Y.Y., Ban, K., Park, H.J., and Lee, J.,-H., Plasmonic colorimetric PCR for Rapid molecular diagnostic assays, Sens. Actuators B, 2021, vol. 337, p. 129762.
Mohammadyousef, P., Paliouras, M., Trifiro, M.A., and Kirk, A.G., Plasmonic and label-free real-time quantitative PCR for point-of-care diagnostics, Analyst, 2021, vol. 146, p. 5619.
Kang, B.-H., Jang, K.-W., Yu, E.-S., Na, H., Lee, Y.-J., Ko, W.-Y., Bae, N.H., Rho, D., and Jeong, K.-H., Ultrafast plasmonic nucleic acid amplification and real-time quantification for decentralized molecular diagnostics, ACS Nano, 2023, vol. 17, p. 6507.
Borzenkov, M., Pallavicini, P., Taglietti, A., D’Alfonso, L., Collini, M., and Chirico, G., Photothermally active nanoparticles as a promising tool for eliminating bacteria and biofilms, Beilstein J. Nanotechnol., 2020, vol. 11, p. 1134.
Akouibaa, A., Masrour, R., Benhamou, M., and Derouiche, A., Thermoplasmonics decontamination of respirators face masks using silver nanoparticles: A new weapon in the fight against COVID-19 pandemic, Plasmonics, 2022, vol. 17, p. 2307.
De Miguel, I., Prieto, I., Albornoz, A., Sanz, V., Weis, C., Turon, P., and Quidant, R., Plasmon-based biofilm inhibition on surgical implants, Nano Lett., 2019, vol. 19, p. 2524.
Milanesi, A., Magni, G., Centi, S., Schifino, G., Aluigi, A., Khlebtsov, B.N., Cavigli, L., Barucci, A., K-hlebtsov, N.G., Ratto, F., Rossi, F., and Pini, R., Optically activated and interrogated plasmonic hydrogels for applications in wound healing, J. Biophotonics, 2020, vol. 13, p. e202000135.
Liu, Y., Li, F., Guo, Z., Xiao, Y., Zhang, Y., Sun, X., Zhe, T., Cao, Y., Wang, L., Lu, Q., and Wang, J., Silver nanoparticle-embedded hydrogel as a photothermal platform for combating bacterial infections, Chem. Eng. J., 2020, vol. 382, p. 122990.
Merkl, P., Zhou, S., Zaganiaris, A., Shahata, M., Eleftheraki, A., Thersleff, T., and Sotiriou, G.A., Plasmonic coupling in silver nanoparticle aggregates and their polymer composite films for near-infrared photothermal biofilm eradication, ACS Appl. Nano Mater., 2021, vol. 4, p. 5330.
Zhao, Y.Q., Sun, Y., Zhang, Y., Ding, X., Zhao, N., Yu, B., Zhao, H., Duan, S., and Xu, F.-J., Well-defined gold nanorod/polymer hybrid coating with inherent antifouling and photothermal bactericidal properties for treating an infected hernia, ACS Nano, 2020, vol. 14, p. 2265.
Nanda, S.S., Wang, T., Hossain, M.I., Yoon, H.Y., Selvan, S.T., Kim, K., and Yi, D.K., Gold-nanorod-based scaffolds for wound-healing applications, ACS Appl. Nano Mater., 2022, vol. 5, p. 8640.
Labouta, H.I., Hooshmand, N., Upreti, T., and El-Sayed, M.A., Localized plasmonic photothermal therapy as a life-saving treatment paradigm for hospitalized COVID-19 patients, Plasmonics, 2021, vol. 16, p. 1029.
Zhuo, Y., Zhang, Y., Wang, B., Cheng, S., Yuan, R., Liu, S., Zhao, M., Xu, B., Zhang, Y., and Wang, X., Gold nanocluster & indocyanine green based triple-effective therapy for MRSA infected central nervous system, Applied Materials Today, 2022, vol. 27, p. 101453.
Mahmoud, N.N., Alkilany, A.M., Khalil, E.A., and Al-Bakri, A.G., Nano-photothermal ablation effect of hydrophilic and hydrophobic functionalized gold nanorods on Staphylococcus aureus and Propionibacterium acnes, Sci. Rep., 2018, vol. 8, p. 6881.
Paithankar, D.Y., Sakamoto, F.H., Farinelli, W.A., Kositratna, G., Blomgren, R.D., Meyer, T.J., Fau-pel, L.J., Kauvar, A.N.B., Lloyd, J.R., Cheung, W.L., Owczarek, W.D., Suwalska, A.M., Kochanska, K.B., Nawrocka, A.K., Paluchowska, E.B., Podolec, K.M., Pirowska, M.M., Wojas-Pelc, A.B., and Ander-son, R.R., Acne treatment based on selective photothermolysis of sebaceous follicles with topically delivered light-absorbing gold microparticles, J. Invest. Dermatol., 2015, vol. 135, p. 1727.
Park, J.W., Shin, S.H., Lee, W.G., Li, K., Seo, S.J., Kim, C.H., and Park, K.Y., Evaluation of the efficacy and safety of the 1064 nm picosecond Nd:YAG laser with a topically applied gold and diamond suspension for facial skin rejuvenation: A pilot study, Dermatol. Ther., 2022, vol. 35, p. e15459.
Seo, J., Roh, H.J., and Jung, J.Y., Gut microbiota modulation and gold nanoparticle-mediated photothermal therapy for treatment of recalcitrant acne, Clin. Case Rep., 2022, vol. 10, p. e05642.
News, U.S. FDA Clears Sebacia Microparticles for the Treatment of Mild to Moderate Inflammatory Acne. www.prnewswire.com/news-releases/us-fda-clears-sebacia-microparticles-for-the-treatment-of-mild-to-moderate-inflammatory-acne-300713403.html.
Masse, F., Ouellette, M., Lamoureux, G., and Boisselier, E., Gold nanoparticles in ophthalmology, Med. Res. Rev., 2019, vol. 39, p. 302.
Sauvage, F., Nguyen, V.P., Li, Y., Harizaj, A., Sebag, J., Roels, D., Van Havere, V., Peynshaert, K., Xiong, R., Fraire, J.C., Tassignon, M.-J., Remaut, K., Paulus, Y.M., Braeckmans, K., and De Smedt, S.C., Laser-induced nanobubbles safely ablate vitreous opacities in vivo, Nat. Nanotechnol., 2022, vol. 17, p. 552.
Lin, Y.-X., Hu, X.-F., Zhao, Y., Gao, Y.-J., Yang, C., Qiao, S.-L., Wang, Y., Yang, P.-P., Yan, J., Sui, X.-C., Qiao, Z.-Y., Li, L.-L., Xie, J.-B., Zhu, S.-Q., Wu, X.-C., Li, Y., Wang, L., and Wang, H., Photothermal ring integrated intraocular lens for high-efficient eye disease treatment, Adv. Mater., 2017, vol. 29, p. 1701617.
Liu, D., Wu, Q., Chen, W., Chen, K., Lin, H., Liu, F., Xie, X., Chen, H.-J., and Chen, W., Nanoporous gold ring-integrated photothermal intraocular lens for active prevention of posterior capsular opacification, Small, 2022, vol. 18, p. 2201098.
Wang, Y., Xu, Z., Li, W., Wei, W., Qin, M., Li, Q., Liu, X., Zhang, X., and Wang, X., A graphene-Ag based near-infrared defined accurate anti-scarring strategy for ocular glaucoma surgery, Biomater. Sci., 2022, vol. 10, p. 1281.
Pang, Y., Wei, C., Li, R., Wu, Y., Liu, W., Wang, F., Zhang, X., and Wang, X., Photothermal conversion hydrogel based mini-eye patch for relieving dry eye with long-term use of the light-emitting screen, Int. J. Nanomed., 2019, vol. 14, p. 5125.
Zare, I., Yaraki, M.T., Speranza, G., Najafa-badi, A.H., Shourangiz-Haghighi, A., Nik, A.B., Manshian, B.B., Saraiva, C., Soenen, S.J., Kogan, M.J., Lee, J.W., Apollo, N.V., Bernardino, L., Araya, E., Mayer, D., Mao, G., and Hamblin, M.R., Gold nanostructures: Synthesis, properties, and neurological applications, Chem. Soc. Rev., 2022, vol. 51, p. 2601.
Pan, W.-T., Liu, P.-M., Ma, D., and Yang, J.-J., Advances in photobiomodulation for cognitive improvement by near-infrared derived multiple strategies, J. Transl. Med., 2023, vol. 21, p. 135.
Wang, Y., Garg, R., Cohen-Karni, D., and Cohen-Karni, T., Neural modulation with photothermally active nanomaterials, Nature Reviews Bioengineering, 2023, vol. 1, p. 193.
Brown, W.G.A., Needham, K., Begeng, J.M., Thompson, A.C., Nayagam, B.A., Kameneva, T., and Stoddart, P.R., Thermal damage threshold of neurons during infrared stimulation, Biomed. Opt. Express, 2020, vol. 11, p. 2224.
Jang, H., Yoon, D., and Nam, Y., Enhancement of thermoplasmonic neural modulation using a gold nanorod-immobilized polydopamine film, ACS Appl. Mater. Interfaces, 2022, vol. 14, p. 24122.
Soloviev, A., Ivanova, I., Sydorenko, V., Sukhano-va, K., Melnyk, M., Dryn, D., and Zholos, A., Calcium-dependent modulation of BKCa channel activity induced by plasmonic gold nanoparticles in pulmonary artery smooth muscle cells and hippocampal neurons, Acta Physiol., 2023, vol. 237, p. e13922.
Begeng, J.M., Tong, W., del Rosal, B., Ibbotson, M., Kameneva, T., and Stoddart, P.R., Activity of retinal neurons can be modulated by tunable near-infrared nanoparticle sensors, ACS Nano, 2023, vol. 17, p. 2079.
Hassanpour-Tamrin, S., Taheri, H., Hasani-Sadrabadi, M.M., Mousavi, S.H.S., Dashtimoghadam, E., Tondar, M., Adibi, A., Moshaverinia, A., Nezhad, A.S., and Jacob, K.I., Nanoscale optoregulation of neural stem cell differentiation by intracellular alteration of redox balance, Adv. Funct. Mater., 2017, vol. 27, p. 1701420.
Qu, A., Sun, M., Kim, J.-Y., Xu, L., Hao, C., Ma, W., Wu, X., Liu, X., Kuang, H., and Kotov, N.A., Stimulation of neural stem cell differentiation by circularly polarized light transduced by chiral nanoassemblies, Nat. Biomed. Eng., 2021, vol. 5, p. 103.
Suthar, J.K., Vaidya, A., and Ravindran, S., Toxic implications of silver nanoparticles on the central nervous system: A systematic literature review, J. Appl. Toxicol., 2023, vol. 43, p. 4.
Pylaev, T., Avdeeva, E.S., and Khlebtsov, N.G., Plasmonic nanoparticles and nucleic acids hybrids for targeted gene delivery, bioimaging, and molecular recognition, J. Innovative Opt. Health Sci., 2021, vol. 14, p. 2130003.
Wang, L., Wei, X., Liu, H., and Fan, Y., Nanomaterial-mediated photoporation for intracellular delivery, Acta Biomater., 2023, vol. 157, p. 24.
Lukianova-Hleb, E.Y., Samaniego, A.P., Wen, J., Metelitsa, L.S., Chang, C.-C., and Lapotko, D.O., Selective gene transfection of individual cells in vitro with plasmonic nanobubbles, J. Controlled Release, 2011, vol. 152, p. 286.
Pylaev, T., Vanzha, E., Avdeeva, E., Khlebtsov, B., and Khlebtsov, N., A novel cell transfection platform based on laser optoporation mediated by Au nanostar layers, J. Biophotonics, 2019, vol. 12, p. e201800166.
Pylaev, T., Efremov, Y., Avdeeva, E.S., Anto-shin, A.A., Shpichka, A.I., Khlebnikova, T.M., Timashev, P., and Khlebtsov, N.G., Optoporation and recovery of living cells under Au nanoparticle layer-mediated NIR-laser irradiation, ACS Appl. Nano Mater., 2021, vol. 4, p. 13206.
Kafshgari, M.H., Agiotis, L., Largilliere, I., Patskovsky, S., and Meunier, M., Antibody-functionalized gold nanostar-mediated on-resonance picosecond laser optoporation for targeted delivery of RNA therapeutics, Small, 2021, vol. 17, p. 2007577.
Yao, C., Rudnitzki, F., He, Y., Zhang, Z., Huttmann, G., and Rahmanzadeh, R., Cancer cell-specific protein delivery by optoporation with laser-irradiated gold nanorods, J. Biophotonics, 2020, vol. 13, p. e202000017.
Nikitin, M.P., Zelepukin, I.V., Shipunova, V.O., Sokolov, I.L., Deyev, S.M., and Nikitin, P.I., Enhancement of the blood-circulation time and performance of nanomedicines via the forced clearance of erythrocytes, Nat. Biomed. Eng., 2020, vol. 4, p. 717.
Gao, H., Zhang, L., Lian, X., Wang, Y., Jiang, S., Wang, G., Dai, X., Zou, H., and Ding, D., A dentin hypersensitivity treatment using highly stable photothermal conversion nanoparticles, Mater. Chem. Front., 2021, vol. 5, p. 3388.
Ribera, J., Vilches, C., Sanz, V., de Miguel, I., Portolés, I., Córdoba-Jover, B., Prat, E., Nunes, V., Jiménes, W., Quidant, R., and Morales-Ruiz, M., Treatment of hepatic fibrosis in mice based on targeted plasmonic hyperthermia, ACS Nano, 2021, vol. 15, p. 7547.
Li, X., Hou, Y., Meng, X., Li, Ge., Xu, F., Teng, L., Sun, F., and Li, Y., Folate receptor-targeting mesoporous silica-coated gold nanorod nanoparticles for the synergistic photothermal therapy and chemotherapy of rheumatoid arthritis, RSC Adv., 2021, vol. 11, p. 3567.
Jiang, Y., Liu, J., Qin, J., Lei, J., Zhang, X., Xu, Z., Li, W., Liu, X., Wang, R., Li, B., and Lu, X., Light-activated gold nanorods for effective therapy of venous malformation, Mater. Today Bio, 2022, vol. 16, p. 100401.
Zhang, X., Cheng, G., Xing, X., Liu, J., Cheng, Y., Ye, T., Wang, Q., Xiao, X., Li, Z., and Deng, H., Near-infrared light-triggered porous AuPd alloy nanoparticles to produce mild localized heat to accelerate bone regeneration, J. Phys. Chem. Lett., 2019, vol. 10, p. 4185.
ACKNOWLEDGMENTS
We are sincerely grateful to V.M. Rudoy (Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences) for useful advices that he gave when this review was written.
Funding
This work was carried out within the framework of the order of the Ministry of Science and Higher Education of the Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dement’eva, O.V., Kartseva, M.E. Noble Metal Nanoparticles in Biomedical Thermoplasmonics. Colloid J 85, 500–519 (2023). https://doi.org/10.1134/S1061933X23700187
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X23700187