Abstract
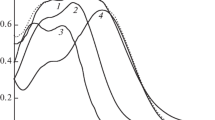
It has been shown that adsorption of natural antioxidants, such as 3-phenylpropenic (cinnamic), 3-(4-hydroxyphenyl)propenic (coumaric), and 3-(3-methoxy-4-hydroxyphenyl)propenic (ferulic) acids, on the surface of cerium dioxide is characterized by S-type isotherms, while adsorption of 3-(3,4-dihydroxyphenyl)propenic (caffeic) acid is described by an H-type isotherm. A linear correlation between adsorption values and hydrophobicity parameter logP has been found in initial regions of the S-type isotherms, with such correlation being typical for parallel orientation of adsorbed molecules relative to an adsorbent surface. The maximum adsorption values (≈2.9 × 10–4 mol/g), as well as dissociation constants (pKCOOH = 4.4–4.6), are almost identical for all acids, thereby suggesting that adsorbate molecules are predominantly bound to the adsorbent surface via carboxyl groups. The presence of hydroxyl groups in molecules of coumaric, ferulic, and caffeic acids widens the рН range of their adsorption as compared with cinnamic acid and promotes their oxidation on the cerium dioxide surface.




Similar content being viewed by others
REFERENCES
Shcherbakov, A.B., Ivanova, O.S., Spivak, N.Ya., Kozik, V.V., and Ivanov, V.K., Sintez i biomeditsinskie primeneniya nanodispersnogo dioksida tseriya (Synthesis and Biomedical Applications of Nanodisperse Cerium Dioxide), Tomsk: Izd. Dom TGU, 2016.
Ould-Moussa, N., Safi, M., Guedeau-Boudeville, M., et al., Nanotoxicology, 2014, vol. 8, p. 799.
Vlasova, N.N., Golovkova, L.P., and Stukalina, N.G., Colloid J., 2015, vol. 77, p. 418.
Vlasova, N.N., Colloid J., 2016, vol. 78, p. 747.
Men’shchikova, E.B., Lankin, V.Z., and Kandalintseva, N.V., Fenol’nye antioksidanty v biologii i meditsine. Stroenie, svoistva, mekhanizmy deistviya (Phenolic Antioxidants in Biology and Medicine: Structure, Properties, Mechanisms of Action), Saarbrücken: LAP LAMBERT Academic, 2012.
Yashin, Ya.I., Ryzhnev, V.Yu., Yashin, A.Ya., and Chernousova, N.I., Prirodnye antioksidanty. Soderzhanie v pishchevykh produktakh i vliyanie ikh na zdorov’e i starenie cheloveka (Nature Antioxidants. Content in Food Products and the Influence on Human Health and Aging), Moscow: TransLit, 2009.
Pokorny, J., EJEAF Chem., 2008, vol. 7, p. 3320.
El-Seedi, H.R., El-Said, A.M., Khalifa, S.A., et al., J. Agric. Food Chem., 2012, vol. 60, p. 10877.
Fizicheskaya khimiya lignina (Physical Chemistry of Lignin), Bogolitsyn, K.G. and Lunin, V.V., Eds., Arkhangel’sk: Arkhangel’sk. Gos. Tekh. Univ., 2009.
Chuev, G.N. and Bazilevskii, M.V., Usp. Khim., 2003, vol. 72, p. 827.
Kulik, T.V., Lipkovska, N.A., Barvinchenko, V.N., et al., J. Colloid Interface Sci., 2009, vol. 339, p. 60.
Kulik, T.V., Barvinchenko, V.N., Lipkovska, N.A., et al., J. Anal. Appl. Pyrolysis, 2011, vol. 90, p. 219.
Kulik, T.V., Lipkovska, N.A., Barvinchenko, V.N., et al., J. Colloid Interface Sci., 2016, vol. 470, p. 132.
Bernshtein, I.Ya. and Kaminskii, Yu.L., Spektrofotometricheskii analiz v organicheskoi khimii (Spectrophotometric Analysis in Organic Chemistry), Leningrad: Khimiya, 1986.
Obshchaya organicheskaya khimiya (General Organic Chemistry), Barton, D. and Ollis, U.D., Eds., Moscow: Khimiya, 1983.
Naseem, S., Laurent, A.D., Carroll, E.C., et al., J. Photochem. Photobiol. A, 2013, vol. 270, p. 43.
Friedman, M. and Jurgens, H.S., J. Agric. Food Chem., 2000, vol. 48, p. 2101.
Linder, P.W., Polyhedron, 1987, vol. 6, p. 53.
Muzafarov, E.N. and Zolotareva, E.K., Biochem. Physiol. Pflanz, 1989, p. 363.
Giles, C.H., Macewan, T.H., Nakhwa, S.N., and Smith, D., J. Chem. Soc., 1960, vol. 10, p. 3973.
http://sis.nlm.nih.gov/chemical.html.
Roche, M., Dufour, C., Mora, N., and Dangles, O., Org. Biomol. Chem., 2005, vol. 3, p. 423.
Dei, A., Gatteschi, D., Sangregorio, C., and Sorace, L., Acc. Chem. Res., 2004, vol. 37, p. 827.
Sever, M.J. and Wilker, J.J., Dalton. Trans., 2004, vol. 7, p. 1061.
ACKNOWLEDGMENTS
This work was supported by the Ukrainian Scientific and Technical Center (project no. P 707) and Sweden Research Council (VR) (contract no. 348-2014-4250).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by L. Tkachenko
Rights and permissions
About this article
Cite this article
Barvinchenko, V.N., Lipkovskaya, N.A., Kulik, T.V. et al. Adsorption of Natural 3-Phenylpropenic Acids on Cerium Dioxide Surface. Colloid J 81, 1–7 (2019). https://doi.org/10.1134/S1061933X19010022
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X19010022