Abstract
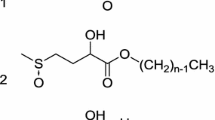
Kinetic regularities are studied for the air oxidation of surfactants that are widely used in the food industry, such as natural phosphatidylcholine (egg lecithin, PC) and synthetic nonionic surfactants Triton X-100 (ТХ-100), Tween 65, and Pluronic F68. Azobis(amidinopropane)-dichloride-initiated oxidation of these surfactants in an aqueous medium at 37°C develops via the chain free-radical mechanism. The chain length is equal to 5–10 units, depending on the initiator-to-surfactant concentration ratio. The rate of surfactant oxidation in an aqueous medium is proportional to the rate of radical initiation. At the same mass concentrations of the reagents, the rate of PC oxidation is several times higher than the oxidation rates of the other surfactants. The addition of TX-100 to PC liposomes decelerates the oxidation; i.e., TX-100 plays the role of an antioxidant for PC. The superposition of the oxidation rates of individual and mixed PC and TX-100 with the sizes of the microaggregates formed in their aqueous solutions shows that the antioxidation action of TX-100 is realized via the formation of a protective layer composed of its ethylene oxide groups, which shields PC liposomes from radicals, which are initiated in the bulk of an aqueous phase due to the decomposition of azobis(amidinopropane) dichloride.
Similar content being viewed by others
References
Emanuel’, N.M. and Lyaskovskaya, Yu.N., Tormozhenie protsessov okisleniya zhirov (Retardation of Fat Oxidation Processes), Moscow Pishcheprodukty, 1961.
Frankel, E.N., Lipid Oxidation, Glasgow Oily, 1998.
Berger, K.G. and Hamilton, R.J., in Developments in Oil and Fats, Hamilton, R.J., Ed., Glasgow: Chapman and Hall, 1995, p. 192.
Coupland, J.N. and McClements, D.J., Trends Food Sci. Technol., 1996, vol. 7, no. 3, p. 83.
Sirota, T.V. and Kasaikina, O.T., Neftekhimiya, 1994, vol. 34, p. 467.
Kasaikina, O.T. Kortenska, V.D., Kartasheva, Z.S., Kuznetsova, G.M., Maximova, T.V., Sirota, T.V., and Yanishlieva, N.V., Colloids Surf. A, 1999, vol. 149, p. 29.
Trunova, N.A., Kartasheva, Z.S., Maksimova, T.V., Bogdanova, Yu.G., and Kasaikina, O.T., Colloid J., 2007, vol. 69, p. 697.
Trunova, N.A., Krugovov, D.A., Bogdanova, Yu.G., and Kasaikina, O.T., Moscow Univ. Chem. Bull., 2008, no. 4, p. 214.
Kasaikina, O.T., Kancheva, V.D., Maximova, T.V., Kartasheva, Z.S., Yanishlieva, N.V., Kondratovich, V.G., and Totseva, I.R., Oxid. Commun., 2006, no. 3, p. 574.
Johnson, L.A., in Food Lipids: Chemistry, Nutrition, and Biotechnology, Akoh, C.C. and Min, D.B., Eds., New York: Marcel Dekker, 2002, p. 206.
Mc’Clements, D.J., Food Emulsions: Principles, Practice and Techniques, Boca Raton: CRC, 2004, p. 2.
Frankel, E.N., Huang, S.W., Kanner, J., and German, J.B., J. Agric. Food Chem., 1994, vol. 42, p. 1054.
Chaiysit, W., Elias, R.J., McClements, D.J., and Decker, E.A., Crit. Rev. Food Sci. Nutrition, 2007, vol. 47, p. 299.
Kasaikina, O.T., Kartasheva, Z.S., and Pisarenko, L.M., Russ. J. Gen. Chem., 2008, vol. 78, p. 1533.
Mengele, E.A., Kartasheva, Z.S., Plashchina, I.G., and Kasaikina, O.T., Colloid J., 2008, vol. 70, p. 753.
Haahr, A.-M. and Jacobsen, C., Eur. J. Lipid Sci. Technol., 2008, vol. 110, p. 949.
Margolis, L.B. and Bergel’son, L.D., Liposomy i ikh vzaimodeistvie s kletkami (Liposomes and Their Interaction with Cells), Moscow Nauka, 1986.
Poste, J., in Liposomes in Biological Systems, Gregoriadis, G., Alisson, A.C., Eds., Chichester: Wiley, 1980, p. 107.
Vladisavljevic, G.T. and Williams, R.A., Adv. Colloid Interface Sci., 2005, vol. 113, p. 1.
Kiyoyama, S., Maruyama, T., Kamiya, N., and Goto, M., J. Microencapsul., 2008, vol. 25, p. 324.
Mengele, E.A., Plashchina, I.G., and Kasaikina, O.T., Colloid J., 2011, vol. 73, p. 815.
Kartasheva, Z.S., Ivanova, N.I., and Kasaikina, O.T, Colloid J., 2006, vol. 68, p. 563.
Roginsky, V.A., Arch. Biochem. Biophys., 2003, vol. 414, p. 261.
Frei, B., Stocker, R., and Ames, B.N., Proc. Natl. Acad. Sci. U. S. A., 1988, vol. 85, p. 9748.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © O.T. Kasaikina, E.A. Mengele, I.G. Plashchina, 2016, published in Kolloidnyi Zhurnal, 2016, Vol. 78, No. 6, pp. 730–734.
Rights and permissions
About this article
Cite this article
Kasaikina, O.T., Mengele, E.A. & Plashchina, I.G. Oxidation of nonionic surfactants with molecular oxygen. Colloid J 78, 767–771 (2016). https://doi.org/10.1134/S1061933X16060065
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X16060065