Abstract
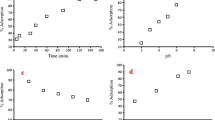
Adsorption kinetics and isotherms for Cd(II) ions on Amberlite IR-120 in a batch process have been studied under different conditions. The influence of various experimental parameters such as the pH, amount of adsorbent material, initial Cd(II) concentration, contact time and solution temperature, on the removal of cadmium ions has been investigated. The experimental isotherms were analyzed using the Langmuir and Freundlich models. The results suggest that the adsorption of Cd(II) ions on Amberlite IR-120 follows the Langmuir model. The adsorption capacities amounted for 250.63, 258.39 and 259.74 mg/g adsorbent at 28, 50 and 70°C, respectively. Two kinetic models were applied to fit the experimental kinetic data. The best fit was obtained with pseudo-second order model. The thermodynamic parameters were also determined. The activation energy calculated from the rate coefficients at different temperatures was 37.45 kJ/mol. The value of the heat of adsorption suggests the endothermic nature of the cation exchange process.
Similar content being viewed by others
References
Sawyer, C.N., McCarty, P.L., and Parkin, G.F., Chemistry for Environmental Engineering and Science, Boston: McGraw-Hill, 2003.
Voegelin, A. and Kretzschmar, R., Eur. J. Soil Sci., 2003, vol. 54, p. 387.
Tsang, D.C.W. and Lo, I.M.C., Environ. Sci. Technol., 2006, vol. 40, p. 6655.
Mohan, D., Singh, K.P., and Singh, V.K., Ind. Eng. Chem. Res., 2005, vol. 44, p. 1027.
Deng, S. and Ting, Y.P., Langmuir, 2005, vol. 21, p. 5940.
Wan, Y., Bao, Y., and Zhou, Q., Chemosphere, 2010, vol. 80, p. 807.
Zhou, D.-M., Wang, Y.-J., Cang, L., Hao, X.-Z., and Luo, X.-S., Chemosphere, 2004, vol. 57, p. 1237.
Mustafa, S., Waseem, M., Naeem, A., Shah, K.H., Ahmad, T., and Hussain, S.Y., Chem. Eng. J., 2010, vol. 157, p. 18.
Han, R., Zou, L., Zhao, X., Xu, Y., Xu, F., Li, Y., and Wang, Y., Chem. Eng. J., 2009, vol. 149, p. 123.
Naeem, A., Woertz, J.R., and Fein, J.B., Environ. Sci. Technol., 2006, vol. 40, p. 5724.
Casqueira, R.G., Torem, M.L., and Kohler, H.M., Miner. Eng., 2006, vol. 19, p. 1388.
Heidmann, I. and Calmano, W., J. Hazard. Mater., 2008, vol. 152, p. 934.
Leybros, A., Roubaud, A., Guichardon, P., and Boutin, O., J. Supercrit. Fluids, 2010, vol. 51, p. 369.
Zagorodni, A.A., Ion Exchange Materials: Properties and Applications, Amsterdam: Elsevier, 2007.
Long, C., Lu, J., Li, A., Hu, D., Liu, F., and Zhang, Q., J. Hazard. Mater., 2008, vol. 150, p. 656.
Gode, F. and Pehlivan, E., J. Hazard. Mater., 2006, vol. 136, p. 330.
Akar, T. and Tunali, S., Miner. Eng., 2005, vol. 18, p. 1099.
Yu, Z., Qi, T., Qu, J., Wang, L., and Chu, J., J. Hazard. Mater., 2009, vol. 167, p. 406.
El-Latif, M.M. and Elkady, M.F., Desalination, 2010, vol. 255, p. 21.
Sakkayawong, N., Thiravetyan, P., and Nakbanpote, W., J. Colloid Interface Sci., 2005, vol. 286, p. 36.
Reddad, Z., Gerente, C., Andres, Y., and Le Cloirec, P., Environ. Sci. Technol., 2002, vol. 36, p. 2067.
Langmuir, I., J. Am. Chem. Soc., 1916, vol. 38, p. 2221.
Freundlich, H.M.F., Z. Phys. Chem., 1906, vol. 57, p. 385.
Gopal, V. and Elango, K.P., J. Hazard. Mater., 2007, vol. 141, p. 98.
Chabani, M., Amrane, A., and Bensmaili, A., Chem. Eng. J., 2006, vol. 125, p. 111.
Lagergren, S., Handlingar, 1898, vol. 24, p. 1.
Shek, T.-H., Ma, A., Lee, V.K.C., and McKay, G., Chem. Eng. J., 2009, vol. 146, p. 63.
Ho, Y.S. and McKay, G., Water Res., 1999, vol. 33, p. 578.
Ho, Y.S. and McKay, G., Water Res., 2000, vol. 34, p. 735.
Sari, A., Tuzen, M., Citak, D., and Soylak, M., J. Hazard. Mater., 2007, vol. 148, p. 387.
Sari, A., Tuzen, M., Citak, D., and Soylak, M., J. Hazard. Mater., 2007, vol. 149, p. 283.
Sari, A., Mendil, D., Tuzen, M., and Soylak, M., Chem. Eng. J., 2008, vol. 144, p. 1.
Donat, R., Akdogan, A., Erdem, E., and Cetisli, H., J. Colloid Interface Sci., 2005, vol. 286, p. 43.
Ajmal, M., Rao, R.A.K., and Khan, M.A., J. Hazard. Mater., 2005, vol. 122, p. 177.
Khani, M.H., Keshtkar, A.R., Ghannadi, M., and Pahlavanzadeh, H., J. Hazard. Mater., 2008, vol. 150, p. 612.
Khezami, L. and Capart, R., J. Hazard. Mater., 2005, vol. 123, p. 223.
Malkoc, E. and Nuhoglu, Y., Sep. Purif. Technol., 2007, vol. 54, p. 291.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Khalil, M., El-Aryan, Y.F. & Abdel-Galil, E.A. Equilibrium studies on the removal of cadmium ion on amberlite IR-120 sorbent. Colloid J 77, 745–753 (2015). https://doi.org/10.1134/S1061933X15060113
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X15060113