Abstract
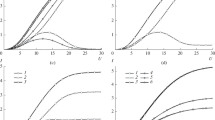
The processes of mass transfer in the metal electrodeposition on a rotating disk electrode from the solution containing three sorts of ions (electroactive metal cation and indifferent electrolyte containing inactive cation and anion) are studied theoretically. The Nernst–Planck equations in the approximation of the solution electroneutrality reduced to a dimensionless form, which takes into account the elecrodiffusion and convective transfer of all types of ions, are used as the mathematical model. The numerical solution of the mathematical model is carried out by the finite volume method using a non-uniform grid. As a result of the numerical solution, the distributions of potential and ion concentrations are obtained with taking into account the interaction between the electric and hydrodynamic fields in the solutions with various concentrations of supporting electrolyte at various diffusion coefficients of ions of all sorts. The dependences of the limiting current of metal electrodeposition on the concentration of supporting electrolyte are obtained. When calculating the limiting current density in the absence of convection, the thickness of the Nernst diffusion layer is calculated taking into account the effective diffusion coefficient of the solution with three sorts of ions at various concentrations of supporting electrolyte. Using several examples with various ratios between the diffusion coefficients of the anion and inactive cation of the electrolyte, the error in the limiting current calculated using the Nernst diffusion layer approximation, as compared with the limiting current obtained taking into account the convective transport of ions, is estimated.













Similar content being viewed by others
REFERENCES
Levich, V.G., Physicochemical Hydrodynamics, Englewood Cliffs, NY: Prentice-Hall, 1962.
Newman, J.S., Electrochemical Systems, Englewood Cliffs, NY: Prentice Hall, 1973.
Ibl, N. and Dossenbach, O., Convective Mass Transport, in Comprehensive Treatise of Electrochemistry, Boston, MA: Springer, 1983, p. 133-237.
Nernst, W., Theorie der reaktionsgeschwindigheit in heterogenen systemen, Z. Phys. Chem., 1904, vol. 47, p. 52.
Brunner, E., Reaktionsgeschwindigheit in heterogenen systemen, Z. Phys. Chem., 1904, vol. 47, p. 56.
Eucken, A., Über den stationären zustand zwischen polarisierten wasserstoffelektroden, Z. Phys. Chem., 1907, vol. 59, no. 1, p. 72.
Aten, A.H.W., Sur l’allure des lignes courant-tension dans l’électrolyse, Recl. Trav. Chim. Pays-Bas, 1923, vol. 42, no. 4, p. 337.
Slendyk, L., Polarographic studies with the dropping mercury cathode. Part XXI. Limiting currents of electrodeposition of metals and of hydrogen, Collect. Czechoslov. Chem. Commun., 1931, vol. 3, p. 385.
Mac Gillavry, D. and Rideal, E.K., On the theory of limiting currents. I. Polarographic limiting currents, Recl. Trav. Chim. Pays-Bas, 1937, vol. 56, no. 10, p. 1013.
Mac Gillavry, D., On the theory of limiting currents: II. Limiting currents of cells without and with an indifferent electrolyte, Recl. Trav. Chim. Pays-Bas, 1937, vol. 56, no. 11, p. 1039.
Mac Gillavry, D., On the theory of limiting currents: III. General solutions with excess of one indifferent electrolyte, Recl. Trav. Chim. Pays-Bas, 1938, vol. 57, no. 1, p. 33.
Hsueh, L. and Newman, J., The role of bisulfate ions in ionic migration effects, Ind. Eng. Chem. Fundamentals., 1971, vol. 10, no. 4, p. 615.
Hornut, J.M., Valentin, G., and Storck, A., The film model for determining the effect of ionic migration in electrochemical systems, J. Appl. Electrochem., 1985, vol. 15, no. 2, p. 237.
Sokirko, A.V., General problem of limiting diffusion-migration currents in a system with ions of three arbitrary charge numbers, J. Electroanal. Chem., 1994, vol. 364, p. 51.
Pritzker, M.D., Steady-state multicomponent diffusion and migration to a planar electrode: Part 1. Analytical solution for the case of a single reacting species, J. Electroanal. Chem. Interfacial Electrochem., 1990, vol. 296, p. 1.
Sokirko, A.V. and Bark, F.H., Diffusion-migration transport in a system with Butler-Volmer kinetics, an exact solution, Electrochim. Acta, 1995, vol. 40, no. 12, p. 1983.
Bortels, L., Van den Bossche, B., Deconinck, J., Vandeputte, S., and Hubin, A., Analytical solution for the steady-state diffusion and migration involving multiple reaction ions. Application to the identification of Butler-Volmer kinetic parameters for the ferri-/ferrocyanide redox couple, J. Electroanal. Chem., 1997, vol. 429, p. 139.
Hicks, M.T. and Fedkiw, P.S., Effects of supporting electrolyte on the mass-transfer limited current for coupled chemical-electrochemical reactions, J. Electroanal. Chem., 1997, vol. 424, p. 75.
Spiegler, K.S., Polarization at ion exchange membrane-solution interfaces. Desalination, 1971, vol. 9, no. 4, p. 367.
Sistat, P. and Pourcelly, G., Steady-state ion transport through homopolar ion-exchange membranes: an analytical solution of the Nernst–Planck equations for a 1 : 1 electrolyte under the electroneutrality assumption, J. Electroanal. Chem., 1999, vol. 460, p. 53.
Volgin, V.M. and Davydov, A.D., Ionic transport through ion-exchange and bipolar membranes, J. Membr. Sci., 2005, vol. 259, p. 110.
Sioda, R.E., Current–potential dependence in the flow electrolysis on a porous electrode, J. Electroanal. Chem., 1972, vol. 34, p. 399.
Trainham, J.A. and Newman, J., A flow-through porous electrode model: application to metal-ion removal from dilute streams, J. Electrochem. Soc., 1977, vol. 124, no. 10, p. 1528.
Milshtein, J.D., Tenny, K.M., Barton, J.L., Drake, J., Darling, R.M., and Brushett, F.R., Quantifying mass transfer rates in redox flow batteries, J. Electrochem. Soc., 2017, vol. 164, no. 11, p. E3265.
Newman, J., Effect of ionic migration on limiting currents, Ind. Eng. Chem. Fundamentals., 1966, vol. 5, no. 4, p. 525.
Newman, J., The effect of migration in laminar diffusion layers, Int. J. Heat Mass Transfer, 1967, vol. 10, no. 7, p. 983.
Wein, O., The effect of migration in laminar diffusion layers, Collect. Czechoslov. Chem. Commun., 1988, vol. 53, no. 4, p. 697.
Malev, V.V. and Durdin, Ya.V., Dependence of limiting current on a rotating disk electrode on concentration of indifferent electrolyte, Elektrokhimiya, 1966, vol. 2, p. 1354.
Kharkats, Yu.I., Calculation of limiting currents as function of base-electrolyte concentration in situation with convective transport, Soviet Electrochem., 1978, vol. 14, p. 984.
Volgin, V.M., Kabanova, T.B., and Davydov, A.D., Theoretical analysis of mass transfer during anodic dissolution of tungsten rotating disk electrode in alkaline solutions, Electrochim. Acta, 2020, vol. 336, Art. 135705.
Nikonenko, V.V., Lebedev, K.A., and Suleimanov, S.S., Influence of the convective term in the Nernst–Planck equation on properties of ion transport through a layer of solution or membrane, Russ. J. Electrochem., 2009, vol. 45, p. 160.
Damaskin, B.B. and Petrii, O.A., Introduction to Electrochemical Kinetics (in Russian), Moscow: Vyssh. Shkola, 1975.
Moukalled, F., Mangani, L., and Darwish, M., The Finite Volume Method in Computational Fluid Dynamics, Cham: Springer, 2016.
Volgin, V.M. and Davydov, A.D., Numerical simulation of steady state ion transfer to rotating disk electrode: accuracy and computational efficiency, J. Electroanal. Chem., 2007, vol. 600, p. 171.
Volgin, V.M. and Davydov, A.D., Effect of migration on homogeneous redox electrocatalysis at rotating disk electrode, Electrochim. Acta, 2018, vol. 259, p. 56.
Funding
The work is supported by the Ministry of Sciences and Higher Education of RF.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by Yu. Pleskov
A tribute to outstanding electrochemist Oleg Aleksandrovich Petrii (1937–2021).
Rights and permissions
About this article
Cite this article
Volgin, V.M., Kabanova, T.B., Andreev, V.N. et al. The Limiting Current of Metal Electrodeposition on Rotating Disk Electrode: The Role of Solution Composition and Transport Properties. Russ J Electrochem 58, 766–780 (2022). https://doi.org/10.1134/S1023193522090154
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193522090154