Abstract
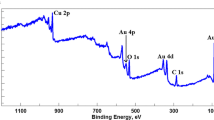
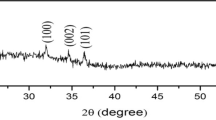
A zinc sulfate film is deposited from aqueous solutions of zinc sulfate onto the gold surface with the aim of preparation of a sensor for electrochemical quartz crystal microbalance (EQCM). The kinetics of this process, the particles formed in solution, and the film itself are studied by the methods of electrochemical quartz crystal microbalance, X-ray photoelectron spectroscopy, transmission electron microscopy, atomic force microscopy, optical and Raman spectroscopies, and dynamic light scattering. The effect of the procedure of gold surface preparation, the reagent concentration, and the temperature on the film adhesion, the length of induction period, the kinetics of film growth, and its structure and thickness are studied. It is shown that the film formation proceeds as a result of deposition of sufficiently coarse 200–700 nm colloid particles of sphalerite. It is demonstrated that this sensor can be used in studying the electrochemical reactions of ZnS and the interface phenomena by the methods of EQCM and cyclic voltammetry.






Similar content being viewed by others
REFERENCES
Tyrakowski, C.M. and Snee, P.T., Ratiometric CdSe/ZnS quantum dot protein sensor, Anal. Chem., 2014, vol. 86, p. 2380.
Dehghani, Z., Nazerdeylami, S., Saievar-Iranizad, E., and Majles Ara, M.H., Synthesis and investigation of nonlinear optical properties of semiconductor ZnS nanoparticles, J. Phys. Chem. Solids., 2011, vol. 2, p. 1008.
Borah, J.P. and Sarma, K.C., Optical and optoelectronic properties of ZnS nanostructured thin film, Acta Phys. Pol., A, 2008, no. 4, vol. 114, p. 713.
Ke, W., Stoumpos, C.C., Logsdon, J.L., Wasielewski, M.R., Yan, Y., Fang, G., and Kanatzidis M.G., TiO2–ZnS cascade electron transport layer for efficient formamidinium tin iodide perovskite solar cells, J. Am. Chem. Soc., 2016, vol. 138, p. 14998.
Mikhlin, Y., Karacharov, A., Vorobyev, S., Romanchenko, A., Likhatski, M., Antsiferova, S., and Markosyan, S., Towards understanding the role of surface gas nanostructures: Effect of temperature difference pretreatment on wetting and flotation of sulfide minerals and Pb–Zn ore, Nanomaterials, 2020, vol. 10, p. 1362.
Mikhlin, Yu., Karacharov, A., Tomashevich, Ye., and Shchukarev, A., Interaction of sphalerite with potassium n-butyl xanthate and copper sulfate solutions studied by XPS of fast-frozen samples and zeta-potential measurement, Vacuum, 2016, vol. 125, p. 98.
Ahlberg, E. and Asbjornsson J., Carbon paste electrodes in mineral processing: an electrochemical study of sphalerite, Hydrometallurgy, 1994, vol. 36, p. 19.
Zhuo, C. and Roe-Hoan, Y., Electrochemistry of copper activation of sphalerite at pH 9.2, Int. J. Miner. Process., 2000, vol. 58, p. 57.
Teng, F., Liu, Q., and Zeng, H., In situ kinetic study of zinc sulfide activation using a quartz crystal microbalance with dissipation (QCM-D), J. Colloid Interface Sci., 2012, vol. 368, no. 1, p. 512.
O’Brien, P. and McAleese, J., Developing an understanding of the processes controlling the chemical bath deposition of ZnS and CdS, J. Mater. Chem., 1998, vol. 8, no. 11, p. 2309.
Doña, J.M., Process and film characterization of chemical-bath-deposited ZnS thin films, J. Electrochem. Soc., 1994, vol. 141, no. 1, p. 205.
Hodes, G., Chemical solution deposition of semiconductor films, New York: Marcel Dekker, 2002.
Agawane, G.L., Kim, S.S., Sung, M., Suryawanshi, M.P., Gurav, K.V., Moholkar, A.V., Lee, J. Y., Ho, Y.J., Patil, P.S., and Hyeok, K.J., Green route fast synthesis and characterization of chemical bath deposited nanocrystalline ZnS buffer layers, Curr. Appl. Phys., 2003, vol. 13, no. 5, p. 850.
Akhtar, M.S., Malik, M.A., Riaz, S., Naseem, S., and O’Brien, P., Optimising conditions for the growth of nanocrystalline ZnS thin films from acidic chemical baths, Mater. Sci. Semicond. Process., 2015, vol. 30, p. 292.
Bayer, A., Boyle, D.S., and O’Brien, P., In situ kinetic studies of the chemical bath deposition of zinc sulfide from acidic solutions, 2002, J. Mater. Chem., 2002, vol. 12, p. 2940.
Sauerbrey, G., Verwendung von schwingquarzen zur wägung dünner schichten und zur mikrowägung, Z. Phys., 1959, vol. 55, no. 2, p. 206.
Hinsberg, W.D., Willson, C.G., and Kanazawa, K.K., Measurement of thin-film dissolution kinetics using a quartz crystal microbalance, J. Electrochem. Soc., 1986, p. 1448.
Mengsu, Y., Thompson, M., and Duncan-Hewitt, W.C., Interfacial properties and the response of the thickness-shear-mode acoustic wave sensor in liquids, Langmuir, 1993, vol. 9, no. 3, p. 802.
Cheng, Y.C., Jin, C.Q., and Gao, F., Raman scattering study of zinc blende and wurtzite ZnS, J. Appl. Phys., 2009, vol. 106, p. 123505.
Mikhlin, Y., X-ray photoelectron spectroscopy in mineral processing studies, Appl. Sci., 2020, vol. 10, p. 5138.
Doyle, R.L. and Lyons, M.E.G., The mechanism of oxygen evolution at superactivated gold electrodes in aqueous alkaline solution, J. Solid State Electrochem., 2014, vol. 18, p. 3271.
Adžić, R.R., Strbac, S., and Anastasijević, N., Electrocatalysis of oxygen on single crystal gold electrodes, Mater. Chem. Phys., 1989, vol. 22, no. 3–4, p. 349.
Burke, L.D., Ahern, A.J., and O’Mullane, A.P., High energy states of gold and their importance in electrocatalytic processes at surfaces and interfaces, Gold Bull., 2002, vol. 35/1, p. 3.
Funding
The study was supported by the Russian Scientific Foundation (grant no. 18-17-00135.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that there is no conflict of interest.
Additional information
Translated by T. Safonova
Rights and permissions
About this article
Cite this article
Krinitsyn, D.O., Romanchenko, A.S., Vorob’ev, S.A. et al. Synthesizing Zinc Sulfide Films on the Gold Surface as the Sensor for Electrochemical Quartz Crystal Microbalance. Russ J Electrochem 57, 1157–1163 (2021). https://doi.org/10.1134/S1023193521120041
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193521120041