Abstract
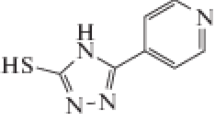
The influence of corrosion inhibition of some thiazole derivatives, namely: 2-(3,5-dimethyl-4-(p-tolyldiazenyl)-1H-pyrazole-1-yl)thiazol-5(4H)-one, 2-(3,5-dimethyl-4(phenyldiazenyl)-1H-pyrazole-1-yl)thiazol-5(4H)-one, and 2-(4-((4-chlorophenyl)diazenyl)-3,5-dimethyl-1H-pyrazole-1-yl)thiazol-5(4H)-one on Zn in 1.0 M HCl solution was studied by utilizing electrochemical and non-electrochemical tests. The obtained data without and with these derivatives were utilized to measure the protection efficiency (η %) which rises with the rise in the concentration of these derivatives. The phenomenon of physical adsorption was proposed and inhibitors adsorption was established to adapt Langmuir adsorption isotherm. Polarization data clearly indicated that these derivatives behaved as mixed inhibitors.












Similar content being viewed by others
REFERENCES
Shimaa, M.A. and Hamedh, A.A., Control of zinc corrosion in acidic media: green fenugreek inhibitor, Trans. Nonferrous Met. Soc. China, 2016, vol. 26, p. 3034.
Hillmana, R., Pickupb, P., Skompska, M., and Vorotyntsev, M.A., Electrochemistry of electroactive materials foreword, Electrochim. Acta, 2014, vol. 122, p. 1.
Fouda, A.S., Aggour, Y., Bekheit, G., and Ismail, M.A., Moringa oleifera extract as a green corrosion inhibitor for zinc in polluted sodium chloride solutions, Int. J. Adv. Res., 2014, vol. 2, p. 1158.
Vashi, R.T. and Bhajiwala, H.M., Ethanolamines as corrosion inhibitors for zinc in (HNO3 + H2SO4) binary acid mixture, E-J. Chem., 2010, vol. 7, no. 2, p. 665.
Mu, G., Tang, L., and Liu, G., Sodium dodecyl benzene sulfonate as a sustainable inhibitor for zinc corrosion in 26% NH4Cl solution, J. Cleaner Prod., 2017, vol. 3, p. 152.
Wang, L., Yunnan, D.S., and Ziran, K., Evaluation of 2-mercapto pyrimidine as corrosion inhibitor for zinc in phosphoric acid solution, Prot. Met., 2001, vol. 23, p. 203.
Ekilik, V.V., Berezhnaya, A.G., and Svyataya, M.N., Inhibition effect of some acridines on the stages of zinc dissolution, Prot. Met., 2001, vol. 37, p. 525.
Rajappa, S.K. and Venkatesha, T.V., Effect of imines on the corrosion rate of zinc in the hydrochloric acid medium by chemical and electrochemical studies, J. Electrochem. Soc. India, 2002, vol. 51, p. 54.
Harned, H.S. and Morrison, J.O., The thermodynamics of hydrochloric acid in dioxane-water mixtures from EMF measurements, I. Standard potentials, J. Am. Chem. Soc., 1936, vol. 58, p. 1908.
Yang Yaohui, Investigation of corrosion inhibition of quaternary ammonium salt on N80 steel in 5 M HCl solution, Int. J. Mater. Sci. Appl., 2017, vol. 6, no. 3, p. 160.
Wang, L., Pu-Jian, X., and Hui-Chun, L., Corrosion inhibition of zinc in phosphoric acid solution by 2-mercaptobenzimidazole, Corros. Sci., 2003 vol. 45, p. 677.
Abdallah, M., Ethoxylated fatty alcohols as corrosion inhibitors for dissolution of zinc in hydrochloric acid, Corros. Sci., 2003, vol. 45, p. 2705.
Emregul Kaan, C. and Atakol, O., Corrosion inhibition of mild steel with Schiff base compounds in 1 M HCl, Mater. Chem. Phys., 2003, vol. 82, p. 188.
Mani, N., Venkatakrishna, S.I., and Bahadur, L., Influence of anions on the inhibition of corrosion of zinc in acidic solutions by pyrrole and its derivatives, Trans. SAEST, 2003, vol. 38, p. 67.
Wang, K., Pickering, H.W., and Weil, K.G., Corrosion inhibition of zinc by benzotriazole with an electrochemical quartz crystal microbalance, J. Electrochem. Soc., 2003, vol. 150, p. B176.
Mani, N., Venkatakrishna, S.I., and Bahadur, L., Influence of anions on the inhibition of corrosion of zinc in acidic solutions by n-heterocyclics, Bull. Electrochem., 2003, vol. 19, p. 53.
Desai, M.N., Talati, J.D., and Shah Neesha, K., Ortho-substituted aniline-N-salicylide as corrosion inhibitors for zinc in sulphuric acid, Indian J. Chem. Sec. A: Inorg. Bio-Inorg. Phys. Theoret. Anal. Chem., 2003, vol. 42, p. 3027.
Mani, N., Venkatakrishna, S.I., and Bahadur, L., Pyridine and its derivatives as inhibitors for the corrosion of zinc in an acidic solution, J. Electrochem. Soc. India, 2003, vol. 52, p. 23.
Desai, M.N.S., Agrawal, Y.K., Talati, J.D., Shah, M.D., and Shah, N.K., Schiff bases of ethylenediamine as corrosion inhibitors of zinc in sulphuric acid, Corros. Sci., 2004, vol. 46, p. 633.
Foad El-Sherbini, E.E., Abdel Wahaab, S.M., and Deyab, M., Ethoxylated fatty acids as inhibitors for the corrosion of zinc in acid media, Mater. Chem. Phys., 2005, vol. 89, p. 183.
Talati, J.D., Desai, M.N., and Shah, N.K., Ortho-, meta-, and para-aminophenol-N-salicylide as corrosion inhibitors of zinc in sulfuric acid, Anti-Corros. Meth. Mater., 2005, vol. 52, p. 108.
Talati, J.D., Desai, M.N., and Shah, N.K., Meta-substituted aniline-N-salicylide as corrosion inhibitors of zinc in sulphuric acid, Mater. Chem. Phys., 2005, vol. 93, p. 54.
Shah, N.K., Desai, M.N., and Talati, J.D., Proc. 10th European Symp. on Corrosion and Scale Inhibitors, Ferrara, 2005, p. 873.
Fouda, A.S., El-Taweel, F.M., and Elgamil, M., Corrosion inhibition of aluminum in hydrochloric acid solution using some pyrazolocarbothioamide derivatives, Int. J. Electrochem. Sci., 2017, vol. 12, p. 11397.
Abdallah, M., Salem, M.M., Al Jahdaly, B.A., Awad, M.I., Helal, E., and Fouda, A.S., Corrosion inhibition of stainless steel type 316 L in 1.0 M HCl solution using 1,3-thiazolidine-5-one derivatives, Int. J. Electrochem. Sci., 2017, vol. 12, p. 4543.
Deyab, M.A., Fouda, A.S., Osman, M.M., and Abdel-Fattah, S., Mitigation of acid corrosion on carbon steel by novel pyrazolone derivatives, RSC Adv., 2017, vol. 7, p. 45232.
Fouda, A.S., Fayed, T., Elmorsi, M.A., and Elsayed, M., Distyryl derivatives as corrosion inhibitors for carbon steel in acid cleaning process in cooling towers, J. Bio- Tribo-Corros., 2017, vol. 3, p. 1.
Fadda, A.A., Abdel-Latif, E., and Youssri, H.M., Synthesis and antioxidant evaluation of new heterocycles derived from p-aminobenzoate ester, J. Mod. Sci. Eng., 2017, vol. 1, p. 45.
Jones, D.A., Principles and Prevention of Corrosion, 2ed., Upper Saddle River, NJ: Prentice Hall, 1983.
Donahue, F.M. and Noor, K., A technique for the evaluation of hydrogen embrittlement characteristics of electroplating baths, J. Electrochem. Soc., 1965, vol. 112, p. 886.
Kamis, E., Bellucci, F., Latanision, R.M., and El-Ashry, E.S., Acid corrosion inhibition of nickel by 2‑(triphenosphoranylidene) succinic anhydride, Corrosion, 1991, vol. 47, p. 677.
Amin, M.A., Abd El-Rehim, S.S., El-Naggar, M.M., and Abd El-Fattah, H.T., Assessment of EFM as a new nondestructive technique for monitoring the corrosion inhibition of low chromium alloy steel in 0.5 M HCl by tyrosine, J. Mater. Sci., 2009, vol. 44, p. 6258.
Oguzie, E.E., Studies on the inhibitive effect of occimium viridis extract on the acid corrosion of mild steel, Mater Chem. Phys., 2006, vol. 99, p. 441.
Fouda, A.S., Mostafa, H.A., Elewady, G.Y., and El-Hashemy, M.A., Low molecular weight straight-chain diamines as corrosion inhibitors for SS in HCl solution, Chem. Eng. Commun., 2008, vol. 195, p. 934.
Kendig, M. and Jeanjaquet, S., Cr(VI) and Ce(III) inhibition of oxygen reduction on copper, J. Electrochem. Soc., 2002, vol. 149, p. B47.
Nicho, M.E., Hu, H., Gonzalez-Rodriguez, J.G., and Salinas, V.M., Protection of stainless steel by polyaniline films against corrosion in aqueous environments, J. Appl. Electrochem., 2006, vol. 36, p. 153.
Mc Cafferty, E. and Hackerman, N., Double layer capacitance of iron and corrosion inhibition with polymethylene diamines, J. Electrochem. Soc., 1972, vol. 119, p. 146.
Shin, H. and Mansfeld, H., A fitting procedure for impedance data of systems with very low corrosion rates, Corros. Sci., 1989, vol. 29, p. 1235.
Martinez, S. and Metikos-Hukovic, M., A nonlinear kinetic model introduced for the corrosion inhibitive properties of some organic inhibitors, J. Appl. Electrochem., 2003, vol. 33, p. 1137.
Lee, C., Yang, W., and Parr, R.G., Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density, Phys. Rev. B, 1988, vol. 37, p. 785.
Issa, R.M., Awad, M.K., and Atlam, F.M., Quantum chemical studies on the inhibition of corrosion of copper surface by substituted uracils, Appl. Surf. Sci., 2008, vol. 255, p. 2433.
Badr, G.E., The role of some thiosemicarbazide derivatives as corrosion inhibitors for C-steel in acidic media, Corros. Sci., 2009, vol. 51, p. 2529.
Mu, G.N., Zhao, T.P., Liu, M., and Gu, T., Effect of metallic cations on corrosion inhibition of an anionic surfactant for mild steel, Corrosion, 1996, vol. 52, p. 853.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Fouda, A.S., Abdel-Latif, E., Helal, H.M. et al. Synthesis and Characterization of Some Novel Thiazole Derivatives and Their Applications as Corrosion Inhibitors for Zinc in 1 M Hydrochloric Acid Solution. Russ J Electrochem 57, 159–171 (2021). https://doi.org/10.1134/S1023193521020105
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193521020105