Abstract
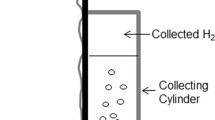
The corrosion behavior of pure (99.999) aluminum in 1 M HCl solution is studied. The regularities of local gas evolution on the surface of test specimen at the open-circuit potential are determined. A number of sites, where hydrogen gas evolves, varies with the time passing through a maximum. The sizes of bubbles prior to their detachment from the specimen surface are determined. The time dependences of gas bubble radius in the course of the bubble growth are obtained. From the experimental results, it is concluded that, at the sites of hydrogen gas evolution, the cathodic reaction prevails, whereas the anodic reaction (aluminum etching) proceeds at the rest specimen surface area. No pits form at the sites of hydrogen evolution during the experiments (up to 5 h). The quantitative analysis of the cathodic polarization curve enabled us to estimate the rate (the corrosion current density) of almost general corrosion after the decay of local gas evolution. The long-term experiments (for 2 months) showed that the pitting corrosion of pure aluminum takes place in 1 M HCl.
Similar content being viewed by others
References
El-Etre, A.Y., Corros. Sci., 2001, vol. 43, p. 1031.
Rethinnagiri, V., Jeyaprakash, P., Arunkumar, M., Maheswaran, V., and Madhiyalagan, A., Adv. Appl. Sci. Res., 2012, vol. 3, p. 1718.
Ali, A.I. and Foaud, N., J. Mater. Environ. Sci., 2012, vol. 3, p. 917.
Branzoi, V., Golgovici, F., and Branzoi, F., Mater. Chem. Phys., 2002, vol. 78, p. 122.
Abiola, O. and Tobun, Y., Chinese Chem. Lett., 2010, vol. 21, p. 1449.
Li, X., Deng, S., and Xie, X., Corros. Sci., 2014, vol. 81, p. 162.
Li, X., Deng, S., and Xie, X., J. Taiwan Inst. Chem. Eng., 2014, vol. 45, p. 1865.
Abd, ElAal E.E., Abd, El., Wanees, S., Farouk, A., Abd, El., and Haleem, S.M., Corros. Sci., 2013, vol. 68, p. 14.
Hassan, R.M. and Zaafarany, I.A., Materials, 2013, vol. 6, p. 2436.
Li, X., Deng, S., and Fu, H., Corros. Sci., 2011, vol. 53, p. 1529.
Abdallah, M., Corros. Sci., 2004, vol. 46, p. 1981.
Akimov, G.V., Stoklitskii, L.I., and Paleolog, E.N., Trudy Instituta fizicheskoi khmii. Vol. III. Issledovaniya po korrozii metallov. No. 2. Novye metody i pribory dlya korrozionnykh ispytanii (Proceedings of Institute of Physical Chemistry. Vol. III. Studies on Metal Corrosion. No. 2. New Methods and Instruments for Corrosion Tests)), Moscow: Akad. Nauk SSSR, 1951.
Curioni, M., Electrochim. Acta, 2014, vol. 120, p. 284.
King, A.D., Birbilis, N., and Scully, J.R., Electrochim. Acta, 2014, vol. 121, p. 394.
Lebouil, S., Duboin, A., Monti, F., Tabeling, P., Volovitch, P., and Ogle, K., Electrochim. Acta, 2014, vol. 124, p. 176.
Yurt, A., Ulutas, S., and Dal, H., Appl. Surf. Sci., 2006, vol. 253, p. 919.
Brett, C.M.A., Corros. Sci., 1992, vol. 33, no. 2, p. 203.
Rybalka, K.V., Beketaeva, L.A., and Davydov, A.D., Russ. J. Electrochem. 2014, vol. 50, p. 108.
Stansbury, E.E. and Buchanan, R.A., Fundamentals of the Electrochemical Corrosion, Materials Park, Ohio: ASM International, 2000.
McCafferty, E., Introduction to Corrosion Science, New York: Springer, 2010.
Mansfeld, F., in Advances in Corrosion Science and Technology, vol. 6, Fontana, G. and Staehle, R.W., Eds., New York: Plenum, 1976, p. 163.
Liu, X., Okafor, P.C., and Zheng, Y.G., Corros. Sci., 2009, vol. 51, p. 744.
Rybalka, K.V., Beketaeva, L.A., and Davydov, A.D., Corros. Sci., 2011, vol. 53, p. P. 630.
Pyun, S.-I. and Lee, W.-J., Corros. Sci., 2001, vol. 43, p. 353.
Nisancioglu, K. and Holtan, H., Werkst. Korros., 1979, vol. 30, p. 105.
Storchai, E.I. and Turkovskaya, A.V., Zashch. Met., 1965, vol. 1, no. 3, p. 293.
Nefedov, V.G., Matveev, V.V., Serebritskii, V.M., Ksenzhek, O.S., and Chikol’ba, T.Yu., Elektrokhimiya, 1991, vol. 27, p. 490.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © K.V. Rybalka, L.A. Beketaeva, A.D. Davydov, 2016, published in Elektrokhimiya, 2016, Vol. 52, No. 5, pp. 522–528.
Rights and permissions
About this article
Cite this article
Rybalka, K.V., Beketaeva, L.A. & Davydov, A.D. Corrosion behavior of aluminum in 1 M HCl solution. Russ J Electrochem 52, 463–469 (2016). https://doi.org/10.1134/S1023193516050104
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193516050104