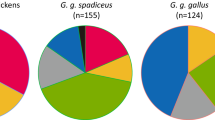Abstract
In this study, we investigated the genetic diversity and phylogeny pattern in five chicken populations from Chongqing, China, using a part of mitochondrial DNA (mtDNA) D-loop sequences. The results revealed that the overall haplotype diversity (Hd) and nucleotide diversity (Pi) among the five chicken populations were 0.91646 and 0.01273, respectively. Forty-one polymorphic sites were identified within the 506-bp length of the mtDNA D-loop sequence in 239 animals. The Neighbor-Joining phylogenetic tree based on FST values demonstrated that the five Chongqing local chicken populations are divided into three clusters. We discovered 35 haplotypes clustered into six mtDNA haplogroups (A, B, C, E, G, and Y). Among the haplogroups, haplogroup A had the highest frequency of approximately 54.3%, whereas haplogroups G and Y had the lowest frequencies of approximately 2.9% each. Furthermore, all the five populations carried multiple haplogroups. Haplogroups A, B and E were shared by all the populations. Mismatch distribution analysis showed that no population expanded, and pairwise comparison of the populations revealed that a significant divergence (p < 0.05) existed among all the populations. The results of this study suggested that all the five chicken populations in Chongqing possessed a rich genetic diversity. The phylogenetic network demonstrated the existence of gene flows from commercial populations. This study provides theoretical basis for the conservation and further utilization of these populations.






Similar content being viewed by others
REFERENCES
Saccone, C., Gissi, C., Lanave, C., et al., Evolution of the mitochondrial genetic system: an overview, Gene, 2000, vol. 261, no. 1, pp. 153–159. https://doi.org/10.1016/s0378-1119(00)00484-4
Gustafsson, C.M., Falkenberg, M., and Larsson, N.G., Maintenance and expression of mammalian mitochondrial DNA, Annu. Rev. Biochem., 2016, vol. 85, pp. 133–160. https://doi.org/10.1146/annurev-biochem-060815-014402
Cann, R.L., Brown, W.M., and Wilson, A.C., Polymorphic sites and the mechanism of evolution in human mitochondrial DNA, Genetics, 1984, vol. 106, no. 3, pp. 479–499. https://doi.org/10.1093/genetics/106.3.479
Elsner, J., Hofreiter, M., Schibler, J., et al., Ancient mtDNA diversity reveals specific population development of wild horses in Switzerland after the Last Glacial Maximum, PLoS One, 2017, vol. 12, no. 5, article E0177458. https://doi.org/10.1371/journal.pone.0177458
Mannen, H., Yonesaka, R., Noda, A., et al., Low mitochondrial DNA diversity of Japanese Polled and Kuchinoshima feral cattle, Anim. Sci. J., 2017, vol. 88, no. 5, pp. 739–744. https://doi.org/10.1111/asj.12716
Ju, Y., Liu, H., He, J., et al., Genetic diversity of Aoluguya reindeer based on D-loop region of mtDNA and its conservation implications, Gene, 2020, vol. 733, article 144271. https://doi.org/10.1016/j.gene.2019.144271
Zhang, J., Jiao, T., and Zhao, S., Genetic diversity in the mitochondrial DNA D-loop region of global swine (Sus scrofa) populations, Biochem. Biophys. Res. Commun., 2016, vol. 473, no. 4, pp. 814–820. https://doi.org/10.1016/j.bbrc.2016.03.125
Bhuiyan, M.S., Chen, S., Faruque, S., et al., Genetic diversity and maternal origin of Bangladeshi chicken, Mol. Biol. Rep., 2013, vol. 40, no. 6, pp. 4123–4128. https://doi.org/10.1007/s11033-013-2522-6
Fathi, M.M., Al-Homidan, I., Motawei, M.I., et al., Evaluation of genetic diversity of Saudi native chicken populations using microsatellite markers, Poult. Sci., 2017, vol. 96, no. 3, pp. 530–536. https://doi.org/10.3382/ps/pew357
Rubin, C.J., Zody, M.C., Eriksson, J., et al., Whole-genome resequencing reveals loci under selection during chicken domestication, Nature, 2010, vol. 464, no. 7288, pp. 587–591. https://doi.org/10.1038/nature08832
Granevitze, Z., Hillel, J., Feldman, M., et al., Genetic structure of a wide-spectrum chicken gene pool, Anim. Genet., 2009, vol. 40, no. 5, pp. 686–693. https://doi.org/10.1111/j.1365-2052.2009.01902.x
Weigend, S. and Romanov, M.N., Molekulare Charakterisierung genetischer Vielfalt beim Geflügel—molecular characterization of genetic diversity in chicken, in Jahresbericht 1998, Braunschweig: Bundesforschungsanstalt für Landwirtschaft (FAL), 1999, p. 66.
Burt, D.W., Chicken genome: current status and future opportunities, Genome Res., 2005, vol. 15, no. 12, pp. 1692–1698. https://doi.org/10.1101/gr.4141805
Hata, A., Nunome, M., Suwanasopee, T., et al., Origin and evolutionary history of domestic chickens inferred from a large population study of Thai red junglefowl and indigenous chickens, Sci. Rep., 2021, vol. 11, no. 1, article 2035. https://doi.org/10.1038/s41598-021-81589-7
Vaarst, M., Steenfeldt, S. and Horsted, K., Sustainable development perspectives of poultry production, Worlds Poult. Sci. J., 2015, vol. 71, no. 4, pp. 609–620. https://doi.org/10.1017/S0043933915002433
Dementieva, N.V., Mitrofanova, O.V., Dysin, A.P., et al., Assessing the effects of rare alleles and linkage disequilibrium on estimates of genetic diversity in the chicken populations, Animal, 2021, vol. 15, no. 3, article 100171. https://doi.org/10.1016/j.animal.2021.100171
Weigend, S., Romanov, M.N., Ben-Ari, G., et al., Overview on the use of molecular markers to characterize genetic diversity in chickens, in World’s Poultry Congress and Exhibition. Book of Abstracts + Participant List and Full Text CD, Istanbul: WPSA—Turkish Branch, 2004, p. 192.
Yang, X., Liu, C.L., Yang, B.G., et al., Investigating genetic diversity and population phylogeny of five Chongqing local chicken populations autosomal using microsatellites, Anim. Biotechnol., 2021, pp. 1–19. https://doi.org/10.1080/10495398.2021.1880421
Xue, Y., Genetic Diversity and Phylogenetic Analyses of Five Chongqing Local Chicken Populations, Chongqing, China: Southwest University, 2021.
Zhao, S.G., Genetic Diversity of mtDNA in Chinese Indigenous Chickens, Lanzhou, China: Gansu Agricultural University, 2006.
Thompson, J.D., Higgins, D.G., and Gibson, T.J., CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice, Nucleic Acids Res., 1994, vol. 22, no. 22, pp. 4673–4680. https://doi.org/10.1093/nar/22.22.4673
Kumar, S., Stecher, G., and Tamura, K., MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for bigger datasets, Mol. Biol. Evol., 2016, vol. 33, no. 7, pp. 1870–1874. https://doi.org/10.1093/molbev/msw054
Huang, X.H., Lin, Y.M., Li, W.N., et al., Complete sequence analysis on the mtDNA control region in WuHua Three-yellow Chicken, J. Jiaying Univ., 2014, vol. 32, no. 2, pp. 58–61.
Rozas, J., Ferrer-Mata, A., Sanchez-Del Barrio, J.C., et al., DnaSP 6: DNA sequence polymorphism analysis of large data sets, Mol. Biol. Evol., 2017, vol. 34, no. 12, pp. 3299–3302. https://doi.org/10.1093/molbev/msx248
Excoffier, L., Laval, G., and Schneider, S., Arlequin (version 3.0): an integrated software package for population genetics data analysis, Evol. Bioinf. Online, 2007, vol. 1, pp. 47–50.
Bandelt, H.J., Forster, P., and Röhl, A., Median-joining networks for inferring intraspecific phylogenies, Mol. Biol. Evol., 1999, vol. 17, pp. 37–48. https://doi.org/10.1093/oxfordjournals.molbev.a026036
Osman, S.A., Yonezawa, T., and Nishibori, M., Origin and genetic diversity of Egyptian native chickens based on complete sequence of mitochondrial DNA D-loop region, Poult. Sci., 2016, vol. 95, no. 6, pp. 1248–1256. https://doi.org/10.3382/ps/pew029
Lyimo, C.M., Weigend, A., Msoffe, P.L., et al., Maternal genealogical patterns of chicken breeds sampled in Europe, Anim. Genet., 2015, vol. 46, no. 4, pp. 447–451. https://doi.org/10.1111/age.12304
Granevitze, Z., Hillel, J., Chen, G.H., et al., Genetic diversity within chicken populations from different continents and management histories, Anim. Genet., 2007, vol. 38, no. 6, pp. 576–583. https://doi.org/10.1111/j.1365-2052.2007.01650.x
Ying, T.S., An analysis of the flora of Qinling Mountain range: its nature, characteristics and origins, Acta Phytotax. Sin., 1994, vol. 32, no. 5, pp. 389–410.
Liu, Y.P., Wu, G.S., Yao, Y.G., et al., Multiple maternal origins of chickens: out of the Asian jungles, Mol. Phylogenet. Evol., 2006, vol. 38, no. 1, pp. 12—19. https://doi.org/10.1016/j.ympev.2005.09.014
Miao, Y.W., Peng, M.S., Wu, G.S., et al., Chicken domestication: an updated perspective based on mitochondrial genomes, Heredity, 2013, vol. 110, no. 3, pp. 277–282. https://doi.org/10.1038/hdy.2012.83
Gongora, J., Rawlence, N.J., Mobegi, V.A., et al., Indo-European and Asian origins for Chilean and Pacific chickens revealed by mtDNA, Proc. Natl. Acad. Sci. U.S.A., 2008, vol. 105, no. 30, pp. 10308–10313. https://doi.org/10.1073/pnas.0801991105
Zhang, T., Liu, H., Yang, L.K., et al., The complete mitochondrial genome and molecular phylogeny of Lueyang black-bone chicken, Br. Poult. Sci., 2018, vol. 59, no. 6, pp. 618–623. https://doi.org/10.1080/00071668.2018.1514581
Jia, X.X., Lu, J.X., Tang, X.J., et al., Genetic diversity of Jiangsu native chicken breeds assessed with the mitochondrial DNA D-loop region, Br. Poult. Sci., 2018, vol. 59, no. 1, pp. 34–39. https://doi.org/10.1080/00071668.2017.1395391
Zhang, T., Du, W., Lu, H., et al., Genetic diversity of mitochondrial DNA of Chinese black-bone chicken, Braz. J. Poult. Sci., 2018, vol. 20, no. 3, pp. 565–572. https://doi.org/10.1590/1806-9061-2018-0739
E, G.X., Hong, Q.H., Zhao, Y.J., et al., Genetic diversity estimation of Yunnan indigenous goat breeds using microsatellite markers, Ecol. Evol., 2019, vol. 9, no. 10, pp. 5916–5924. https://doi.org/10.1002/ece3.5174
Funding
This work was supported by the Innovation Team Building Program in Chongqing universities (CXTDG201602004).
Author information
Authors and Affiliations
Contributions
X.Y., G.X.E., B.G.Y., C.L.L., Y.G., and Y.G. contributed equally to this study. M.H.L., G.X.E., X.Y., C.L.L., B.G.Y., H.Q.H., Y.M.H., W.Y.Z., D.P.Z., Y.Y. and B.E.C. conceived and designed the experiments. X.Y., Y.G., B.G.Y., and Y.G. analyzed the data. G.X.E. and X.Y. wrote the paper. M.H.L. provided funding. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflicts of interest.
Statement on the welfare of animals. The animal experiment was approved by the Laboratory Animal Ethics Committee Southwest University.
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Yang, X., E, GX., Yang, BG. et al. Genetic Diversity and Phylogeny Pattern across Chongqing (China) Chicken Populations Using mtDNA D-Loop Sequences. Russ J Genet 58, 1007–1016 (2022). https://doi.org/10.1134/S1022795422080117
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795422080117