Abstract
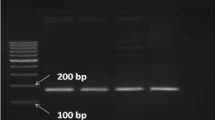
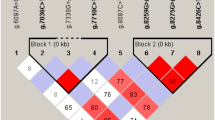
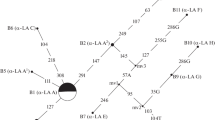
Beta-lactoglobulin (β-LG) is the major whey protein associated with lactation traits in ruminants and has a potential impact on human health. So far, the polymorphisms and molecular characteristics of buffalo LGB gene have not been well understood. In this study, the complete coding sequence (CDS) of buffalo LGB were isolated and its polymorphisms were detected using direct sequencing of PCR product. The CDS of LGB gene for river and swamp buffalo was 543 nucleotides in length, encoding a peptide with 180 amino acid residues. A total of eight single nucleotide polymorphisms (SNPs) was identified in the CDS of LGB gene in two types of buffalo. Among them, c.46G>A and c.131C>T were non-synonymous, which led to an amino acid substitution from Ala to Thr at the 16th position of the signal peptide and a p.Ala26Val substitution in the mature peptide of buffalo β-LG, respectively. The prediction showed that the p.Ala26Val may affect the function of buffalo β-LG. Seven LGB haplotypes were defined based on the SNPs observed in buffalo, and accordingly, two variants and two synonymous variants of buffalo β-LG were inferred, named variant A, A1, A2 and B, respectively. All the variants determined in buffalo did not exist in the animals of Bos genus. The sequence alignment of β-LG mature peptide showed that there were two different amino acids (p.Ile1Leu and p.Val162Ile) between buffalo and the animals of Bos genus. The signal peptide of buffalo β-LG was two amino acids longer than that of Bos genus and there was an differential amino acid at the 13th position of signal peptide, which may lead to different functions of signal peptide between buffalo and Bos genus. The hydrophilicity of buffalo β-LG variants A and B were slightly different, and their physicochemical characteristics were similar to those of cattle β-LG B. The mature peptides of β-LG in buffalo and Bos genus had a lipocalin domain, which revealed that they had the same function. This study will give us new insights into the variation, physicochemical characteristics and biological function of buffalo LGB gene.





Similar content being viewed by others
REFERENCES
Meza-Nieto, M.A., Vallejo-Cordoba, B., González-Córdova, A.F., et al., Effect of beta-lactoglobulin A and B whey protein variants on the rennet-induced gelation of skim milk gels in a model reconstituted skim milk system, J. Dairy Sci., 2007, vol. 90, no. 2, pp. 582—593.
Liu, H.C., Chen, W.L., and Mao, S.J.T., Antioxidant nature of bovine milk β-lactoglobulin, J. Dairy Sci., 2007, vol. 90, no. 2, pp. 547—555.
Kontopidis, G., Holt, C., and Sawyer, L., Invited review: β-lactoglobulin: binding properties, structure, and function, J. Dairy Sci., 2004, vol. 87, no. 4, pp. 785—796.
Hasni, I., Bourassa, P., and Tajmir-Riahi, H., Binding of cationic lipids to milk β-lactoglobulin, J. Phys. Chem. B, 2011, vol. 115, no. 20, pp. 6683—6690.
Li, H., Wei, J., Dong, Y., et al., Interaction between 2-(p-toluidino)-6-naphthalenesulfonic acid sodium salt (TNS) and β-lactoglobulin, Can. J. Chem., 2016, vol. 94, no. 8, pp. 680—686.
Zheng, G., Liu, H., Zhu, Z., et al., Selenium modification of β-lactoglobulin (β-Lg) and its biological activity, Food Chem., 2016, vol. 204, pp. 246—251.
Hernández-Ledesma, B., Recio, I., and Amigo, L., β‑Lactoglobulin as source of bioactive peptides, Amino Acids, 2008, vol. 35, no. 2, pp. 257—265.
Zheng, G., Xu, X., Zheng, J., et al., Protective effect of seleno-β-lactoglobulin (Se-β-lg) against oxidative stress in D-galactose-induced aging mice, J. Funct. Foods, 2016, vol. 27, pp. 310—318.
Farrell, H.M., Jimenez-Flores, R., Bleck, G.T., et al., Nomenclature of the proteins of cows’ milk–sixth revision, J. Dairy Sci., 2004, vol. 87, no. 6. pp. 1641—1674.
Caroli, A.M., Chessa, S., and Erhardt, G.J., Invited review: milk protein polymorphisms in cattle: effect on animal breeding and human nutrition, J. Dairy Sci., 2009, vol. 92, no. 11, pp. 5335—5352.
Karimi, K., Nasiri, M.T.B., Fayyazi, J., et al., Allele and genotype frequencies of β-lactoglobulin gene in Iranian Najdi cattle and buffalo populations using PCR-RFLP, Afr. J. Biotechnol., 2009, vol. 8, no. 15, pp. 3654—3657.
Miluchová, M., Trakovická, A., and Gábor, M., Analysis of beta-lactoglobuline gene (LGB) polymorphism in different breeds of bulls by high resolution melting, Scientific Papers: Animal Science and Biotechnologies, 2012, vol. 45, no. 1, pp. 193—197.
Michelizzi, V.N., Dodson, M.V., Pan, Z., et al., Water buffalo genome science comes of age, Int. J. Biol. Sci., 2010, vol. 6, no. 4, pp. 333—349.
Basilicata, M., Pepe, G., Adesso, S., et al., Antioxidant properties of buffalo-milk dairy products: Aβ-Lg peptide released after gastrointestinal digestion of buffalo ricotta cheese reduces oxidative stress in intestinal epithelial cells, Int. J. Mol. Sci., 2018, vol. 19, no. 7, p. 1955.
Ambrosio, C.D., Arena, S., Salzano, A.M., et al., A proteomic characterization of water buffalo milk fractions describing PTM of major species and the identification of minor components involved in nutrient delivery and defense against pathogens, Proteomics, 2008, vol. 8, no. 17, pp. 3657—3666.
Song, S., Ou-Yang, Y., Huo, J., et al., Molecular cloning, sequence characterization, and tissue expression analysis of three water buffalo (Bubalus bubalis) genes— ST6GAL1, ST8SIA4, and SLC35C1, Arch. Anim. Breed., 2016, vol. 59, pp. 363—372.
Yeh, F.C. and Boyle, T.B.J., Population genetic analysis of co-dominant and dominant marker and quantitative traits, Belg. J. Bot., 1997, vol. 129, pp. 157—163.
Kumar, S., Stecher, G., and Tamura, K., MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets, Mol. Biol. Evol., 2016, vol. 33, no. 7, pp. 1870—1874.
Stephens, M., Smith, N.J., and Donnelly, P., A new statistical method for haplotype reconstruction from population data, Am. J. Hum.Genet., 2001, vol. 68, no. 4, pp. 978—989.
Choi, Y. and Chan, A.P., PROVEAN web server: a tool to predict the functional effect of amino acid substitutions and indels, Bioinformatics, 2015, vol. 31, no. 16, pp. 2745—2747.
Chen, F., Li, Q., Gu, M., et al., Identification of a mutation in FGF23 involved in mandibular prognathism. Sci. Rep., 2015, vol. 5, no. 1, p. 11250.
Pellegrini, A., Dettling, C., Thomas, U., et al., Isolation and characterization of four bactericidal domains in the bovine β-lactoglobulin, Biochim. Biophys. Acta, Gen. Subj., 2001, vol. 1526, no. 2, pp. 131—140.
Hernández-Ledesma, B., Dávalos, A., Bartolomé, B., et al., Preparation of antioxidant enzymatic hydrolysates from α-lactalbumin and β-lactoglobulin: identification of active peptides by HPLC-MS/MS. J. Agric. Food Chem., 2005, vol. 53, no. 3, pp. 588—593.
Funding
This work was supported by the National Natural Science Foundation of China (nos. 31760659 and 31460582), the Natural Science Foundation Key Project of Yunnan Province, China (no. 2014FA032).
Author information
Authors and Affiliations
Contributions
X.Y. Fan and W.B. Guo contributed equally to this work.
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. The procedures for sample collection were performed in accordance with the Guide for Animal Care and Use of Experimental Animals and approved by the Institutional Animal Care and Use Committee of Yunnan Agricultural University (Kunming, Yunnan, China).
Rights and permissions
About this article
Cite this article
Fan, X.Y., Guo, W.B., Qiu, L.H. et al. Polymorphisms, Molecular Characteristics of LGB Gene in River and Swamp Buffalo (Bubalus bubalis). Russ J Genet 57, 204–212 (2021). https://doi.org/10.1134/S1022795421020034
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795421020034