Abstract
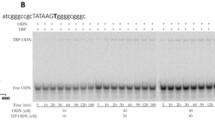
Atherosclerosis (AS) and AS-related pathologies such as coronary heart disease, myocardial infarction, angina pectoris, and stroke are the leading causes of death in the world. The atherogenesis can be influenced by many factors, in particular, overweight, hypertension, diabetes, hyperlipoproteinemia, and other diseases in anamnesis, as well as genetic predisposition, which may be due to single nucleotide polymorphisms (SNPs) in some cases. In this work, we studied only those regions of promoters of the human protein-coding genes where SNP markers of changes in the affinity of the TATA-binding protein (TBP) for these promoters have already been associated with the atherosclerosis-related pathologies. As a result, within the dbSNP database, we found those unannotated SNPs which change such affinity just as the known biomedical SNP markers do here (according to the predictions made by our Web service SNP_TATA_Z-tester, http://wwwmgs.bionet.nsc.ru/cgi-bin/mgs/tatascan_fox/start.pl). For example, the known SNP marker rs35036378 of the high risks of the primary pT1 tumor reduces the TBP affinity for the ESR2 gene promoter and, thus, the estrogen receptor β abundancy in blood, which is a known physiological marker of calcification of blood vessels in atherogenesis. Near this known SNP marker, we found an unannotated SNP rs766797386, which can also reduce the TBP affinity for the same promoter and, thus, decrease the abundance of the estrogen receptor β in blood. Thus, we propose rs766797386 as a candidate SNP marker for accelerated atherogenesis due to calcification of blood vessels. The possibility of using a diet of natural food with high abundance of calcium (Ca), which is recommended by nutritionists to slow down calcification in the case when individuals have SNP markers of accelerated calcification, is discussed in contrast to the use of Ca-enriched nutritional supplements that can cause the opposite effect. In the same way, a total of 33 candidate SNP markers were predicted and discussed to accelerate or slow down atherogenesis. After clinical verification, the candidate SNP markers predicted by this work can help those who would like to slow down atherogenesis through lifestyle corrections using their genome sequencing data.


Similar content being viewed by others
REFERENCES
Barquera, S., Pedroza-Tobias, A., Medina, C., et al., Global overview of the epidemiology of atherosclerotic cardiovascular disease, Arch. Med. Res., 2015, vol. 46, no. 5, pp. 328—338. https://doi.org/10.1016/j.arcmed.2015.06.006
Napoli, C., D’Armiento, F.P., Mancini, F.P., et al., Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia: intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions, J. Clin. Invest., 1997, vol. 100, no. 11, pp. 2680—2690. https://doi.org/10.1172/JCI119813
Li, A.C. and Glass, C.K., The macrophage foam cell as a target for therapeutic intervention, Nat. Med., 2002, vol. 8, no. 11, pp. 1235—1242. https://doi.org/10.1038/nm1102-1235
Lusis, A.J., Atherosclerosis, Nature, 2000, vol. 407, no. 6801, pp. 233—241. https://doi.org/10.1038/35025203
Hirayama, S., Soda, S., Ito, Y., et al., Circadian change of serum concentration of small dense LDL-cholesterol in type 2 diabetic patients, Clin. Chim. Acta., 2010, vol. 411, nos. 3—4, pp. 253—257. https://doi.org/10.1016/j.cca.2009.11.017
Lathe, R., Sapronova, A., and Kotelevtsev, Y., Atherosclerosis and Alzheimer—diseases with a common cause? Inflammation, oxysterols, vasculature, BMC Geriatr., 2014, vol. 14, p. 36. https://doi.org/10.1186/1471-2318-14-36
Glass, C.K. and Witztum, J.L., Atherosclerosis: the road ahead, Cell, 2001, vol. 104(4), pp. 503—516. https://doi.org/10.1016/S0092-8674(01)00238-0
Telenti, A., Pierce, L.C., Biggs, W.H., et al., Deep sequencing of 10 000 human genomes, Proc. Natl. Acad. Sci. U.S.A., 2016, vol. 113, no. 42, pp. 11901—11906. https://doi.org/10.1073/pnas.1613365113
Sherry, S.T., Ward, M.H., Kholodov, M., et al., dbSNP: the NCBI database of genetic variation, Nucleic Acids Res., 2001, vol. 29, no. 1, pp. 308—311. https://doi.org/10.1093/nar/29.1.308
Varzari, A., Deyneko, I.V., Tudor, E., and Turcan, S., Polymorphisms of glutathione S-transferase and methylenetetrahydrofolate reductase genes in Moldavian patients with ulcerative colitis: genotype—phenotype correlation, Meta Gene, 2016, vol. 7, pp. 76—82. https://doi.org/10.1016/j.mgene.2015.12.002
Trovato, G.M., Sustainable medical research by effective and comprehensive medical skills: overcoming the frontiers by predictive, preventive and personalized medicine, EPMA J., 2014, vol. 5, no. 1, p. 14. https://doi.org/10.1186/1878-5085-5-14
Amberger, J.S., Bocchini, C.A., Schiettecatte, F., et al., OMIM.org: Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders, Nucleic Acids Res., 2015, vol. 43, pp. D789—D798. https://doi.org/10.1093/nar/gku1205
Zerbino, D.R., Wilder, S.P., Johnson, N., et al., The Ensembl Regulatory Build, Genome Biol., 2015, vol. 16, p. 56. https://doi.org/10.1186/s13059-015-0621-5
Ponomarenko, M., Mironova, V., Gunbin, K., and Savinkova, L., Hognessbox, in Brenner’s Encyclopedia of Genetics, San Diego: Academic, 2013, vol. 3, pp. 491—494. https://doi.org/10.1016/B978-0-12-374984-0.00720-8
Martianov, I., Viville, S., and Davidson, I., RNA polymerase II transcription in murine cells lacking the TATA binding protein, Science, 2002, vol. 298, no. 5595, pp. 1036—1039. https://doi.org/10.1126/science.1076327
Mogno, I., Vallania, F., Mitra, R.D., and Cohen, B.A., TATA is a modular component of synthetic promoters, Genome Res., 2010, vol. 20, no. 10, pp. 1391—1397. https://doi.org/10.1101/gr.106732.110
Ponomarenko, M.P., Rasskazov, D.A., Arkova, O.V., et al., How to use SNP_TATA_Comparator to find a significant change in gene expression caused by the regulatory SNP of this gene’s promoter via a change in affinity of the TATA-binding protein for this promoter, Biomed. Res. Int., 2015, vol. 2015, p. 359835. https://doi.org/10.1155/2015/359835
Arkova, O.V., Ponomarenko, M.P., Rasskazov, D.A., et al., Obesity-related known and candidate SNP markers can significantly change affinity of TATA-binding protein for human gene promoters, BMC Genomics, 2015, vol. 16, suppl. 13, p. S5. https://doi.org/10.1186/1471-2164-16-S13-S5
Chadaeva, I.V., Ponomarenko, M.P., Rasskazov, D.A., et al., Candidate SNP markers of aggressiveness-related complications and comorbidities of genetic diseases are predicted by a significant change in the affinity of TATA-binding protein for human gene promoters, BMC Genomics, 2016, vol. 17, suppl. 14, p. 995. https://doi.org/10.1186/s12864-016-3353-3
Chadaeva, I.V., Ponomarenko, M.P., Rasskazov, D.A., et al., Candidate SNP markers of reproductive potential are predicted by a significant change in the affinity of TATA-binding protein for human gene promoters, BMC Genomics, 2018, vol. 19, suppl. 3, p. 0. https://doi.org/10.1186/s12864-018-4478-3
Ponomarenko, M., Arkova, O., Rasskazov, D., et al., Candidate SNP markers of gender-biased autoimmune complications of monogenic diseases are predicted by a significant change in the affinity of TATA-binding protein for human gene promoters, Front. Immunol., 2016, vol. 7, p. 130. https://doi.org/10.3389/fimmu.2016.00130
Ponomarenko, P., Rasskazov, D., Suslov, V., et al., Candidate SNP markers of chronopathologies are predicted by a significant change in the affinity of TATA-binding protein for human gene promoters, BioMed. Res. Int., 2016, vol. 2016, p. 8642703. https://doi.org/10.1155/2016/8642703
Ponomarenko, M., Arkova, O., Rasskazov, D., et al., Candidate SNP markers of familial and sporadic Alzheimer’s diseases are predicted by a significant change in the affinity of TATA-binding protein for human gene promoters, Front. Aging Neurosci., 2017, vol. 9, p. 231. https://doi.org/10.3389/fnagi.2017.00231
Plengpanich, W., Le Goff, W., Poolsuk, S., et al., CETP deficiency due to a novel mutation in the CETP gene promoter and its effect on cholesterol efflux and selective uptake into hepatocytes, Atherosclerosis, 2011, vol. 216, no. 2, pp. 370—373. https://doi.org/10.1016/j.atherosclerosis.2011.01.051
Philips, S., Richter, A., Oesterreich, S., et al., Functional characterization of a genetic polymorphism in the promoter of the ESR2 gene, Horm. Cancer, 2012, vol. 3, no. 1—2, pp. 37—43. https://doi.org/10.1007/s12672-011-0086-2
McRobb, L.S., McGrath, K.C.Y., Tsatralis, T., et al., Estrogen receptor control of atherosclerotic calcification and smooth muscle cell osteogenic differentiation, Arterioscler. Thromb. Vasc. Biol., 2017, vol. 37, no. 6, pp. 1127—1137. https://doi.org/10.1161/atvbaha.117.309054
Anderson, J.J., Kruszka, B., Delaney, J.A., et al., Calcium intake from diet and supplements and the risk of coronary artery calcification and its progression among older adults: 10-year follow-up of the Multi-Ethnic Study of Atherosclerosis (MESA), J. Am. Heart Assoc., 2016, vol. 5, no. 10. e003815. https://doi.org/10.1161/jaha.116.003815
Ceneri, N., Zhao, L., Young, B.D., et al., Rac2 modulates atherosclerotic calcification by regulating macrophage interleukin-1β production, Arterioscler. Thromb. Vasc. Biol., 2017, vol. 37, no. 2, pp. 328—340. https://doi.org/10.1161/atvbaha.116.308507
Landrum, M.J., Lee, J.M., Riley, G.R., et al., ClinVar: public archive of relationships among sequence variation and human phenotype, Nucleic Acids Res., 2014, vol. 42, pp. D980—D985. https://doi.org/10.1093/nar/gkt1113
Cao, W., Ning, J., Yang, X., and Liu, Z., Excess exposure to insulin is the primary cause of insulin resistance and its associated atherosclerosis, Curr. Mol. Pharmacol., 2011, vol. 4, no. 3, pp. 154—166. https://doi.org/10.2174/1874467211104030154
Liu, Z., Zhou, Z., Huang, G., et al., Long-term effects intensive medical therapy on the development and progression of subclinical atherosclerosis and the metabolic syndrome in Chinese patients with type 2 diabetes mellitus, Medicine (Baltimore), 2016, vol. 95, no. 46. e5201. https://doi.org/10.1097/md.0000000000005201
Niemann, S., Broom, W.J., and Brown, R.H. Jr., Analysis of a genetic defect in the TATA box of the SOD1 gene in a patient with familial amyotrophic lateral sclerosis, Muscle Nerve, 2007, vol. 36, no. 5, pp. 704—707. https://doi.org/10.1002/mus.20855
Rafael, H., David, J.O., and Vilca, A.S., Etiology and treatment of amyotrophic lateral sclerosis, Am. J. Neurodegener. Dis., 2017, vol. 6, no. 1, pp. 1—8.
Savinkova, L., Drachkova, I., Arshinova, T., et al., An experimental verification of the predicted effects of promoter TATA-box polymorphisms associated with human diseases on interactions between the TATA boxes and TATA-binding protein, PLoS One, 2013, vol. 8, no. 2. e54626. https://doi.org/10.1371/journal.pone.0054626
Watanabe, M., Zingg, B.C., and Mohrenweiser, H.W., Molecular analysis of a series of alleles in humans with reduced activity at the triosephosphate isomerase locus, Am. J. Hum. Genet., 1996, vol. 58, no. 2, pp. 308—316.
Vives-Corrons, J.L., Rubinson-Skala, H., Mateo, M., et al., Triosephosphate isomerase deficiency with hemolytic anemia and severe neuromuscular disease: familial and biochemical studies of a case found in Spain, Hum. Genet., 1978, vol. 42, no. 2, pp. 171—180.
Balla, G., Vercellotti, G., Eaton, J.W., and Jacob, H.S., Heme uptake by endothelium synergizes polymorphonuclear granulocyte-mediated damage, Trans. Assoc. Am. Physicians, 1990, vol. 103, pp. 174—179.
Kioumourtzoglou, M.A., Seals, R.M., Gredal, O., et al., Cardiovascular disease and diagnosis of amyotrophic lateral sclerosis: a population based study, Amyotroph. Lateral Scler. Frontotemporal. Degener., 2016, vol. 17, nos. 7—8, pp. 548—554. https://doi.org/10.1080/21678421.2016.1208247
Arnaud, E., Barbalat, V., Nicaud, V., et al., Polymorphisms in the 5' regulatory region of the tissue factor gene and the risk of myocardial infarction and venous thromboembolism: the ECTIM and PATHROS studies, Arterioscler. Thromb. Vasc. Biol., 2000, vol. 20, no. 3, pp. 892—898. https://doi.org/10.1161/01.atv.20.3.892
Hasenstab, D., Lea, H., Hart, C.E., et al., Tissue factor overexpression in rat arterial neointima models thrombosis and progression of advanced atherosclerosis, Circulation, 2000, vol. 101, no. 22, pp. 2651—2657. https://doi.org/10.1161/01.cir.101.22.2651
Kavlie, A., Hiltunen, L., Rasi, V., and Prydz, H., Two novel mutations in the human coagulation factor VII promoter, Thromb. Haemost., 2003, vol. 90, no. 2, pp. 194—205. https://doi.org/10.1160/th02-09-0050
Zacharski, L.R., Delprete, S.A., Kisiel, W., et al., Atherosclerosis and coronary bypass surgery in hereditary factor VII deficiency, Am. J. Med., 1988, vol. 84, no. 5, pp. 955—959. https://doi.org/10.1016/0002-9343(88)90078-2
Martiney, J.A., Cerami, A., and Slater, A.F., Inhibition of hemozoin formation in Plasmodium falciparum trophozoite extracts by heme analogs: possible implication in the resistance to malaria conferred by the beta-thalassemia trait, Mol. Med., 1996, vol. 2, no. 2, pp. 236—246.
Wang, H., Luo, W., Wang, J., et al., Paradoxical protection from atherosclerosis and thrombosis in a mouse model of sickle cell disease, Br. J. Haematol., 2013, vol. 162, no. 1, pp. 120—129. https://doi.org/10.1111/bjh.12342
Starodubtseva, N.L., Sobolev, V.V., Soboleva, A.G., et al., Genes expression of metalloproteinases (MMP-1, MMP-2, MMP-9, and MMP-12) associated with psoriasis, Russ. J. Genet., 2011, vol. 47, no. 9, article 1117. https://doi.org/10.1134/S102279541109016X
Hunninghake, G.M., Cho, M.H., Tesfaigzi, Y., et al., MMP12, lung function, and COPD in high-risk populations, N. Engl. J. Med., 2009, vol. 361, no. 27, pp. 2599—2608. https://doi.org/10.1056/nejmoa0904006
Manetti, M., Ibba-Manneschi, L., Fatini, C., et al., Association of a functional polymorphism in the matrix metalloproteinase-12 promoter region with systemic sclerosis in an Italian population, J. Rheumatol., 2010, vol. 37, no. 9, pp. 1852—1857. https://doi.org/10.3899/jrheum.100237
Motterle, A., Xiao, Q., Kiechl, S., et al., Influence of matrix metalloproteinase-12 on fibrinogen level, Atherosclerosis, 2012, vol. 220, no. 2, pp. 351—354. https://doi.org/10.1016/j.atherosclerosis.2011.11.003
Boldt, A., Culpi, L., Tsuneto, L., et al., Diversity of the MBL2 gene in various Brazilian populations and the case of selection at the mannose-binding lectin locus, Hum. Immunol., 2006, vol. 67, no. 9, pp. 722—734. https://doi.org/10.1016/j.humimm.2006.05.009
Sziller, I., Babula, O., Hupuczi, P., et al., Mannose-binding lectin (MBL) codon 54 gene polymorphism protects against development of pre-eclampsia, HELLP syndrome and pre-eclampsia-associated intrauterine growth restriction, Mol. Hum. Reprod., 2007, vol. 13, no. 4, pp. 281—285. https://doi.org/10.1093/molehr/gam003
Cervera, A., Planas, A.M., Justicia, C., et al., Genetically-defined deficiency of mannose-binding lectin is associated with protection after experimental stroke in mice and outcome in human stroke, PLoS One, 2010, vol. 5, no. 2, p. e8433. https://doi.org/10.1371/journal.pone.0008433
Losin, I.E., Shakhnovich, R.M., Zykov, K.A., and Ruda, M.Ya., Cardiovascular diseases and the mannose-binding lectin, Kardiologiya, 2014, vol. 54, no. 3, pp. 64—70.
Saita, E., Kondo, K., and Momiyama, Y., Anti-inflammatory diet for atherosclerosis and coronary artery disease: antioxidant foods, Clin. Med. Insights Cardiol., 2015, vol. 8, suppl. 3, pp. 61—65. https://doi.org/10.4137/cmc.S17071
Burgner, D., Rockett, K., Ackerman, H., et al., Haplotypic relationship between SNP and microsatellite markers at the NOS2A locus in two populations, Genes Immun., 2003, vol. 4, no. 7, pp. 506—514. https://doi.org/10.1038/sj.gene.6364022
Clark, I.A., Rockett, K.A., and Burgner, D., Genes, nitric oxide and malaria in African children, Trends Parasitol., 2003, vol. 19, no. 8, pp. 335—337. https://doi.org/10.1016/S1471-4922(03)00147-8
Gonzalez-Martinez, J.A., Moddel, G., Ying, Z., et al., Neuronal nitric oxide synthase expression in resected epileptic dysplastic neocortex, J. Neurosurg., 2009, vol. 110, no. 2, pp. 343—349. https://doi.org/10.3171/2008.6.17608
Zhao, J.F., Shyue, S.K., Lin, S.J., et al., Excess nitric oxide impairs LXR(α)-ABCA1-dependent cholesterol efflux in macrophage foam cells, J. Cell Physiol., 2014, vol. 229, no. 1, pp. 117—125. https://doi.org/10.1002/jcp.24429
Matsunaga, A., Sasaki, J., Han, H., et al., Compound heterozygosity for an apolipoprotein A1 gene promoter mutation and a structural nonsense mutation with apolipoprotein A1 deficiency, Arterioscler. Thromb. Vasc. Biol., 1999, vol. 19, no. 2, pp. 348—355. https://doi.org/10.1161/01.atv.19.2.348
van Capelleveen, J.C., Kootte, R.S., Hovingh, G.K., and Bochem, A.E., Myocardial infarction in a 36-year-old man with combined ABCA1 and APOA-1 deficiency, J. Clin. Lipidol., 2015, vol. 9, no. 3, pp. 396—399. https://doi.org/10.1016/j.jacl.2015.01.006
Parolini, C., Bjorndal, B., Busnelli, M., et al., Effect of dietary components from Antarctic krill on atherosclerosis in apoE-deficient mice, Mol. Nutr. Food Res., 2017, vol. 61, no. 12. https://doi.org/10.1002/mnfr.201700098
Nalls, M.A., Wilson, J.G., Patterson, N.J., et al., Admixture mapping of white cell count: genetic locus responsible for lower white blood cell count in the Health ABC and Jackson Heart studies, Am. J. Hum. Genet., 2008, vol. 82, no. 1, pp. 81—87. https://doi.org/10.1016/j.ajhg.2007.09.003
Michon, P., Woolley, I., Wood, E.M., et al., Duffy-null promoter heterozygosity reduces DARC expression and abrogates adhesion of the P. vivax ligand required for blood-stage infection, FEBS Lett., 2001, vol. 495, nos. 1—2, pp. 111—114. https://doi.org/10.1016/S0014-5793(01)02370-5
Wan, W., Liu, Q., Lionakis, M.S., et al., Atypical chemokine receptor 1 deficiency reduces atherogenesis in ApoE-knockout mice, Cardiovasc. Res., 2015, vol. 106, no. 3, pp. 478—487. https://doi.org/10.1093/cvr/cvv124
Zhang, Y., Proenca, R., Maffei, M., et al., Positional cloning of the mouse obese gene and its human homologue, Nature, 1994, vol. 372, no. 6505, pp. 425—432. https://doi.org/10.1038/372425a0
Skrypnik, K., Suliburska, J., Skrypnik, D., et al., The genetic basis of obesity complications, Acta Sci. Pol. Technol. Aliment., 2017, vol. 16, no. 1, pp. 83—91. https://doi.org/10.17306/j.afs.2017.0442
Chiba, T., Shinozaki, S., Nakazawa, T., et al., Leptin deficiency suppresses progression of atherosclerosis in apoE-deficient mice, Atherosclerosis, 2008, vol. 196, no. 1, pp. 68—75. https://doi.org/10.1016/j.atherosclerosis.2007.01.040
Jun, J.Y., Ma, Z., Pyla, R., and Segar, L., Leptin treatment inhibits the progression of atherosclerosis by attenuating hypercholesterolemia in type 1 diabetic Ins2(+/Akita):apoE(-/-) mice, Atherosclerosis, 2012, vol. 225, no. 2, pp. 341—347. https://doi.org/10.1016/j.atherosclerosis.2012.10.031
Hubbard, R., Kosch, C.L., Sanchez, A., et al., Effect of dietary protein on serum insulin and glucagon levels in hyper- and normocholesterolemic men, Atherosclerosis, 1989, vol. 76, no. 1, pp. 55—61. https://doi.org/10.1016/0021-9150(89)90193-7
Suslov, V.V., Ponomarenko, P.M., Ponomarenko, M.P., et al., TATA box polymorphisms in genes of commercial and laboratory animals and plants associated with selectively valuable traits, Russ. J. Genet., 2010, vol. 46, no. 4, pp. 394—403. https://doi.org/10.1134/S1022795410040022
Hahn, S., Buratowski, S., Sharp, P.A., and Guarente, L., Yeast TATA-binding protein TFIID binds to TATA elements with both consensus and nonconsensus DNA sequences, Proc. Natl. Acad. Sci. U.S.A., 1989, vol. 86, no. 15, pp. 5718—5722. https://doi.org/10.1073/pnas.86.15.5718
Ponomarenko, P.M., Savinkova, L., Drachkova, I., et al., A step-by-step model of TBP/TATA box binding allows predicting human hereditary diseases by single nucleotide polymorphism, Dokl. Biochem. Biophys., 2008, vol. 419, no. 1, pp. 88—92. https://doi.org/10.1134/S1607672908020117
Bucher, P., Weight matrix descriptions of four eukaryotic RNA polymerase II promoter elements derived from 502 unrelated promoter sequences, J. Mol. Biol., 1990, vol. 212, no. 4, pp. 563—578. https://doi.org/10.1016/0022-2836(90)90223-9
Ponomarenko, M.P., Ponomarenko, J.V., Frolov, A.S., et al., Identification of sequence-dependent features correlating to activity of DNA sites interacting with proteins, Bioinformatics, 1999, vol. 15, no. 7—8, pp. 687—703. https://doi.org/10.1093/bioinformatics/15.7.687
Karas, H., Knuuppel, R., Schulz, W., et al., Combining structural analysis of DNA with search routines for the detection of transcription regulatory elements, Comput. Appl. Biosci., 1996, vol. 12, no. 5, pp. 441—446.
Kimura, M., Evolutionary rate at the molecular level, Nature, 1968, vol. 217, no. 5129, pp. 624—626. https://doi.org/10.1038/217624a0
Suslov, V.V., Ponomarenko, P.M., Efimov, V.M., et al., SNPs in the HIV-1 TATA box and the AIDS pandemic, J. Bioinf. Comput. Biol., 2010, vol. 8, no. 3, pp. 607—625. https://doi.org/10.1142/S0219720010004677
Waardenberg, A.J., Basset, S.D., Bouveret, R., and Harvey, R.P., CompGO: an R package for comparing and visualizing gene ontology enrichment differences between DNA binding experiments, BMC Bioinf., 2015, vol. 16, p. 275. https://doi.org/10.1186/s12859-015-0701-2
Ponomarenko, M., Rasskazov, D., Chadaeva, I., et al., SNP_TATA_Comparator: genomewide landmarks for preventive personalized medicine, Front. Biosci., 2017, vol. 9, no. 2, pp. 276—306. https://doi.org/10.2741/s488
Haeussler, M., Raney, B.J., Hinrichs, A.S., et al., Navigating protected genomics data with UCSC genome browser in a box, Bioinformatics, 2015, vol. 31, no. 5, pp. 764—766. https://doi.org/10.1093/bioinformatics/btu712
Lu, Z., PubMed and beyond: a survey of web tools for searching biomedical literature, Database (Oxford), 2011, vol. 2011, p. baq036. https://doi.org/10.1093/database/baq036
Denis, A., Mergaert, L., Fostier, C., et al., Orphan diseases and orphan medicines: a Belgian and European study, J. Pharm. Belg., 2009, vol. 86, no. 4, pp. 131—137.
Rubbert-Roth, A., Orphan diseases in rheumatology: exemplified by polyarthritis nodosa, Z. Rheumatol., 2012, vol. 71, no. 2, pp. 119—121. https://doi.org/10.1007/s00393-011-0902-7
Brewer, G.J., Drug development for orphan diseases in the context of personalized medicine, Transl. Res., 2009, vol. 154, no. 6, pp. 314—322. https://doi.org/10.1016/j.trsl.2009.03.008
Drachkova, I., Savinkova, L., Arshinova, T., et al., The mechanism by which TATA-box polymorphisms associated with human hereditary diseases influence interactions with the TATA-binding protein, Hum. Mutat., 2014, vol. 35, no. 5, pp. 601—608. https://doi.org/10.1002/humu.22535
Arkova, O., Kuznetsov, N., Fedorova, O., and Savinkova, L., A real-time study of the interaction of TBP with a TATA box-containing duplex identical to an ancestral or minor allele of human gene LEP or TPI, J. Biomol. Struct. Dyn., 2017, vol. 35, no. 14, pp. 3070—3081. https://doi.org/10.1080/07391102.2016.1241190
Ponomarenko, P.M., Suslov, V.V., Savinkova, L., et al., A precise equation of equilibrium of four steps of TBP binding with the TATA box for prognosis of phenotypic manifestation of mutations, Biophysics (Moscow), 2010, vol. 55, no. 3, pp. 358—369. https://doi.org/10.1134/S0006350910030036
Ponomarenko, P.M., Ponomarenko, M.P., Drachkova, I.A., et al., Prediction of the affinity of the TATA-binding protein to TATA boxes with single nucleotide polymorphisms, Mol. Biol. (Moscow), 2009, vol. 43, no. 3, pp. 472—479. https://doi.org/10.1134/S0026893309030157
Deplancke, B., Alpern, D., and Gardeux, V., The genetics of transcription factor DNA binding variation, Cell, 2016, vol. 166, no. 3, pp. 538—554. https://doi.org/10.1016/j.cell.2016.07.012
Gunbin, K.V., Ponomarenko, M.P., Suslov, V.V., et al., Evolution of brain active gene promoters in human lineage towards the increased plasticity of gene regulation, Mol. Neurobiol., 2018, vol. 55, no. 3, pp. 1871—1904. https://doi.org/10.1007/s12035-017-0427-4
Wu, J., Wu, M., Li, L., et al., dbWGFP: a database and web server of human whole-genome single nucleotide variants and their functional predictions, Database (Oxford), 2016, vol. 2016, p. baw024. https://doi.org/10.1093/database/baw024
Haldane, J.B.S., The cost of natural selection, J. Genet., 1957, vol. 55, no. 3, pp. 511—524.
Funding
The text of this paper was written by M.P. Ponomarenko with the support of the Ministry of Education and Science of the Russian Federation within the Program for Enhancing the Competitiveness of Leading Russian Universities among the World’s Leading Research and Education Centers (project 5-100); the concept and the study design (N.A. Kolchanov) were supported by the Integration Project of the Siberian Branch of the Russian Academy of Sciences no. 0324-2018-0021; and the Web service update (D.A. Rasskazov, I.V. Chadaeva, and E.A. Oshchepkova) and data analysis (E.B. Sarypova, I.A. Drachkova, and L.K. Savinkova) were supported by projects no. 0324-2019-0040 and no. 0324-2019-0042, respectively, from the Russian Government Budget.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any research using animals as an object. This article does not contain any research involving people as an object.
Additional information
Translated by K. Lazarev
Rights and permissions
About this article
Cite this article
Ponomarenko, M.P., Rasskazov, D.A., Chadaeva, I.V. et al. Candidate SNP Markers of Atherosclerosis That May Significantly Change the Affinity of the TATA-Binding Protein for the Human Gene Promoters. Russ J Genet 55, 1137–1151 (2019). https://doi.org/10.1134/S1022795419090114
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795419090114