Abstract
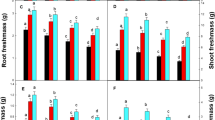
Under the conditions of a growing experiment, the authors studied the effect of zinc at concentrations of 5 (control), 50, 100, and 150 mg/kg substrate on growth, the intensity of lipid peroxidation (LPO), and the activity of the components of the antioxidant system in Brassica juncea L. (Сzern.) variety Slavyanka and Sinapis alba L. cultivar Belgium plants. Some differences and similarities were found in the AOS response of the studied species to an excess of zinc in the root environment. Thus, there were no changes in the intensity of lipid peroxidation in B. juncea under the influence of zinc in high concentrations, despite the high content of the metal in the roots and shoots. At the same time, even in the presence of metal at a concentration of 50 mg/kg substrate, an increase in the activity of guaiacol peroxidase (GPX) and catalase was observed. In S. alba at high concentrations of zinc in the substrate, the metal content in the shoots was higher than in B. juncea. At the same time, the content of malondialdehyde noticeably increased, despite the increased activity of superoxide dismutase and GPX. In both studied plant species, an increase in the zinc concentration in the substrate to 50 mg/kg and above led to an increase in the level of proline, while the content of carotenoids decreased. Considering that, in the studied concentrations, the metal had a less strong negative effect on shoot growth in B. juncea compared with S. alba, it was concluded that plants of this species are more resistant to excess zinc in the root environment.

Similar content being viewed by others
REFERENCES
Mourato, P.M., Moreira, I.N., Leitão, I., Pinto, F.R., Sales, J.R., and Martins, L.L., Effect of heavy metals in plants of the genus Brassica, IJMS, 2015, vol. 16, p. 17975. https://doi.org/10.3390/ijms160817975
Zeremski, T., Ranđelović, D., Jakovljevic, K., Jeromela, A.M., and Milić, S., Brassica species in phytoextractions: real potentials and challenges, Plants, 2021, vol. 10, p. 2340. https://doi.org/10.3390/plants10112340
Bortoloti, G.A. and Baron, D., Phytoremediation of toxic heavy metals by Brassica plants: A biochemical and physiological approach, Environ. Adv., 2022, vol. 8, p. 100204. https://doi.org/10.1016/j.envadv.2022.100204
Małecka, A., Konkolewska, A., Hanć, A., Barałkiewicz, L.C., Ratajczak, E., Staszak, A.M., Kmita, H., and Jarmuszkiewicz, W., Insight into the phytoremediation capability of Brassica juncea (v. Malopolska): metal accumulation and antioxidant enzyme activated, IJMS, 2019, vol. 20, p. 4355. https://doi.org/10.3390/ijms20184355
Balafrej, H., Bogusz, D., Triqui, Z.A. Guedira, A., Bendaou, N., Smouni, A., and Fahr, M., Zinc hyperaccumulation in plants: a review, Plants, 2020, vol. 9, p. 562. https://doi.org/10.3390/plants9050562
Xu, J., Chai, T., Zhang, Y., Lang, M., and Han, L., The cation-efflux transporter BjCET2 mediates zinc and cadmium accumulation in Brassica juncea L. leaves, Plant Cell Rep., 2009, vol. 28, p. 1235. https://doi.org/10.1007/s00299-009-0723-1
Xu, J., Zhang, Y.X., Wei, W., Han, L., Guan, Z.Q., Wang, Z., and Chai, T.Y., BjDHNs confer heavy-metal tolerance in plants, Mol. Biotechnol., 2008, vol. 38, p. 91. https://doi.org/10.1007/s12033-007-9005-8
Khan, M.I.R., Jahan, B., Alajmi, M.F., Rehman, M.T., and Khan, N.A., Exogenously sourced ethylene modulates defense mechanisms and promotes tolerance to zinc stress in mustard (Brassica juncea L.), Plants, 2019, vol. 8, p. 540. https://doi.org/10.3390/plants8120540
Prasad, K.V.S.K., Saradhi, P.P., and Sharmila, P., Concerted action of antioxidant enzymes and curtailed growth under zinc toxicity in Brassica juncea, Environ. Exp. Bot., 1999, vol. 42, p. 1.
Feigl, G., Kolbert, Z., Lehotai, N., Molnár, Á., Ördög, A., Bordé, Á., Laskay, G., and Erdei, L., Different zinc sensitivity of Brassica organs is accompanied by distinct responses in protein nitration level and patternet, Ecotoxicol. Environ. Saf., 2016, vol. 125, p. 141. https://doi.org/10.1016/j.ecoenv.2015.12.006
Du, X., Zeng, T., Feng, Q., Hu, L., Luo, X., Weng, Q., He, J., and Zhu, B., The complete chloroplast genome sequence of yellow mustard (Sinapis alba L.) and its phylogenetic relationship to other Brassicaceae specie set, Gene, 2020, vol. 731, p. 144340. https://doi.org/10.1016/j.gene.2020.144340
Stanis£awska-Glubiak, E. and Karzeniowska, J., Tolerance of white mustard (Sinapsis alba L.) to soil pollution with several heavy metals, Ecol. Chem. Eng. A., 2001, vol. 8, p. 445.
Zalewska, M. and Nogalska, A., Phytoextraction potential of sunflower and white mustard plants in zinc-contaminated soil, Chil. J. Agric. Res., 2014, vol. 74, p. 485. https://doi.org/10.4067/S0718-58392014000400016
Soleimannejad, Z., Sadeghipour, H.R., Abdolzadeh, A., and Golalipour, M., Physiological responses of white mustard grown in Zn-contaminated soilset, Acta Physiol. Plant., 2020, vol. 42, p. 131. https://doi.org/10.1007/s11738-020-03119-8
Juskulak, M., Grobelak, A., Grosser, A., and Vandenbulcke, F., Gene expression, DNA damage and other stress markers in Sinapis alba L. exposed to heavy metals with special reference to sewage sludge application on contaminated sites, Ecotoxicol. Environ. Saf., 2019, vol. 181, p. 508. https://doi.org/10.1016/j.ecoenv.2019.06.025
Juskulak, M., Grobelak, A., and Vandenbulcke, F., Effects of sewage sludge supplementation on heavy metal accumulation and the expression of ABC transporters in Sinapis alba L. during assisted phytoremediation of contaminated sites, Ecotoxicol. Environ. Saf., 2020, vol. 197, p. 110606. https://doi.org/10.1016/j.ecoenv.2020.110606
Sharama, Sh.S., Dietz and K.J., The relationship between metal toxicity and cellular redox imbalance, Trends Plant Sci., 2008, vol. 14, p. 43. https://doi.org/10.1016/j.tplants.2008.10.007
Wang, Ch., Zhang, S.H., Wang, P.F., Hou, J., Zhang, W.J., Li, W., and Lin, Zh.P., The effect of excess Zn on mineral nutrition and antioxidative response in rapeseed seedlings, Chemosphere, 2009, vol. 75, p. 1468. https://doi.org/10.1016/j.chemosphere.2009.02.033
Yang, H., Zhang, J., and Li, J., Physiological response to zinc pollution of rape (Brassica chinensis L) in paddy soil ecosystem, Adv. Mater., 2011, vol. 356, p. 39. https://doi.org/10.4028/www.scientific.net/AMR.356-360.39
Blasco, B., Graham, N.S., and Broadley, M.R., Antioxidant response and carboxylate metabolism in Brassica rapa exposed to different external Zn, Ca, and Mg supply, J. Plant Physiol., 2015, vol. 176, p. 16. https://doi.org/10.1016/j.jplph.2014.07.029
Du, J., Guo, Zh., Li, R., Ali, A., Guo, D., Lahori, A.H., Wang, P., Liu, X., Wang, X., and Zhang, Z., Screening of Chinese mustard (Brassica juncea L.) cultivars for the phytoremediation of Cd and Zn based on the plant physiological mechanisms, Environ. Pollut., 2020, vol. 216, p. 114213. https://doi.org/10.1016/j.envpol.2020.114213
Stewart, R.C. and Bewley, J.D., Lipid peroxidation associated with accelerated aging of soybean axes, Plant Physiol., 1980, vol. 65, p. 245. https://doi.org/10.1104/pp.65.2.245
Beauchamp, Ch. and Fridovich, I., Superoxide dismutase improved assays and an assay applicable to acrylamide gels, Anal. Biochem., 1971, vol. 44, p. 276.
Ershova, M.A., Nikerova, K.M., Galibina, N.A., Sofronova, I.N., and Borodina, M.N., Some minor characteristics of spectrophotometric determination of antioxidant system and phenolic metabolism enzyme activity in wood plant tissues of Pinus sylvestris L, Protein Pept. Lett., 2022, vol. 9, p. 711. https://doi.org/10.2174/0929866529666220414104747
Aebi, H., Catalase in vitro, Methods in Enzymol., 1984, vol. 105, p. 121.
Maehly, A.C., The assay of catalase and peroxidase, Meth. Biochem. Anal., 1954, vol. 1, p. 357.
Bradford, M.M., A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem., 1976, vol. 72, p. 248.
Bates, L.S., Waldren, R.P., and Teare, I.D., Rapid determination of free proline for water-stress studies, Plant Soil., 1973, vol. 39, p. 205.
Wintermans, J.E.G., and De Mots, A., Spectrophotometric characteristics of chlorophyll a and b and their phaeophytins in ethanol, Biochim. Biophys. Acta, 1965, vol. 109, p. 448. https://doi.org/10.1016/0926-6585(65)90170-6
NSAM No. 499-AES/MS. Determination of the elemental composition of rocks, soils, soils and bottom sediments by atomic emission with inductively coupled plasma and mass spectral with inductively coupled plasma methods (ed. 2015).
Natasha, N., Shanid, M., Bibi, I., Iqbal, J., Khalid, S., Murtaza, B., Bakhat, H.F., Farooq, A.B.U., Amjad, M., Hammad, H.M., Niazi, N.Kh., and Arshad, M., Zinc in soil-plant-human system: A data-analysis review, Sci. Total Environ., 2022, vol. 808, p. 152024. https://doi.org/10.1016/j.scitotenv.2021.152024
Alia Prasad, K.V.S.K. and Saradhi, P.P., Effect of zinc on free radicals and proline in Brassica and Cajanus, Phytochem., 1995, vol. 39, p. 45. https://doi.org/10.1016/0031-9422(94)00919-K
Mittler, R., Oxidative stress, antioxidants and stress tolerance, Trends Plant Sci., 2002, vol. 7, p. 405.
Schutzendubel, A. and Polle, A., Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhizatin, J. Exp. Bot., 2002, vol. 53, p. 1351.
Dai, H., Wei, Sh., Skuza, L., and Jia, G., Selenium spiked in soil promoted zinc accumulation of Chinese cabbage and improved its antioxidant system and lipid peroxidation, Ecotoxicol. Environ. Saf., 2019, vol. 180, p. 179. https://doi.org/10.1016/j.ecoenv.2019.05.017
Srivastava, M., Ma, L.Q., Singh, N., and Singh, Sh., Antioxidant responses of hyper-accumulator and sensitive fern species to arsenic, J. Exp. Bot., 2005, vol. 56, p. 1335. https://doi.org/10.1093/jxb/eri134
Mohmoud, A., Elgawad, H.A., Hamed, B.A., Beemster, G.T.S., and El-Shafey, N.M., Differences incadmium accumulation, detoxification and antioxidant defenses between contrasting maize cultivars implicate a role of superoxide dismutase in Cd tolerance, Antioxidants, 2021, vol. 10, p. 1812. https://doi.org/10.3390/antiox10111812
Ghnaya, A.B., Hourmant, A., Cerantola, S., Kervarec, N., Cabon, J.Y., Branchard, M., and Charles, G., Influence of zinc on soluble carbohydrate and free amino acid levels in rapeseed plants regenerated in vitro in the presence of zinc, Plant Cell, Tissue Organ Cult., 2010, vol. 102, p. 191. https://doi.org/10.1007/s11240-010-9721-9
Lin, M.Z. and Jin, M.F., Soil Cu contamination destroys the photosynthetic systems and hampers the growth of green vegetables, Photosynthetica, 2018, vol. 56, p. 1336. https://doi.org/10.1007/s11099-018-0831-7
Funding
The study was supported by a grant from the Russian Science Foundation (project no. 22-24-00668). The equipment of the Center for Collective Use of the Federal Research Center Karelian Scientific Center, Russian Academy of Sciences, was used in the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving humans as research subjects.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Nilova, I.A., Repkina, N.S. & Kaznina, N.M. Influence of Excess Zinc on the Activity of Components of the Antioxidant System in Brassica juncea L. (Czern.) and Sinapis alba L. Plants. Russ J Plant Physiol 70, 96 (2023). https://doi.org/10.1134/S1021443723600861
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S1021443723600861