Abstract
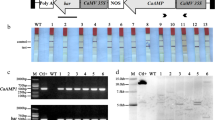
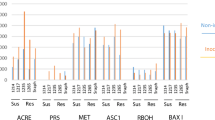
The genome of Stellaria media contains a gene family for hevein-like antimicrobial peptides, some of which are known to encode two peptides released from the translation product as a result of post-translational proteolysis. These peptides have been shown to inhibit the growth of bacteria and fungi, including potato pathogens Alternaria solani and Alternaria alternata. One of these genes, ProSmAMP1, was introduced into the potato genome under the control of the light-inducible promoter of Cab gene from common wheat. The resulting transgenic lines expressed ProSmAMP1 mRNA during several vegetative passages, and their resistance to early blight was assessed by several indicators of detached leaf infection, with plants having the highest expression of the transgene also showing the highest resistance.




Similar content being viewed by others
REFERENCES
Schepers, H., Hausladen, H., and Hansen, J.G., Epidemics and control of early and late blight, 2017 and 2018 in Europe, Proceedings of the Seventeenth EuroBlight Workshop, 2019, vol. 19, p. 11. https://agro.au.dk/fileadmin/ euroblight/Workshops/Proceedings/Special_Report_19_ Totaal_LR.pdf.
Gravesen, S., Fungi as a cause of allergic disease, Allergy, 1979, vol. 34, p. 135. https://doi.org/10.1111/J.1398-9995.1979.TB01562.X
Tsedaley, B., Review on early blight (Alternaria spp.) of potato disease and its management options, J. Biol. Agricul. Healthcare, 2014, vol. 4, p. 191. https://www.iiste. org/Journals/index.php/JBAH/article/view/18650.
Adolf, B., Andrade-Piedra, J., Bittara Molina, F., Przetakiewicz, J., Hausladen, H., Kromann, P., Lees, A., Lindqvist-Kreuze, H., Perez, W., and Secor, G.A., Fungal, oomycete, and plasmodiophorid diseases of potato, The Potato Crop: Its Agricultural, Nutritional and Social Contribution to Humankind, 2019, vol. 9, p. 307. https://doi.org/10.1007/978-3-030-28683-5_9
Van Der Waals, J.E., Korsten, L., and Aveling, T.A.S., A review of early blight of potato, Afr. Plant Prot., 2001, vol. 7, p. 1.
Kumar, C. A., Yadav, J., Kumar, G. A., and Gupta, K., Integrated disease management of early blight (Alternaria Solani) of potato, Trop. Agrobiodiv., 2021, vol. 2, p. 77. https://doi.org/10.26480/trab.02.2021.77.81
Shinde, B.A., Dholakia, B.B., Hussain, K., Panda, S., Meir, S., Rogachev, I., Aharoni, A., Giri, A.P., and Kamble, A.C., Dynamic metabolic reprogramming of steroidal glycol-alkaloid and phenylpropanoid biosynthesis may impart early blight resistance in wild tomato (Solanum arcanum Peralta), Plant Mol. Biol., 2017, vol. 95, p. 411. https://doi.org/10.1007/S11103-017-0660-2/FIGURES/7
Roddick, J.G. and Rijnenberg, A.L., Effect of steroidal glycoalkaloids of the potato on the permeability of liposome membranes, Physiol. Plant, 1986, vol. 68, p. 436. https://doi.org/10.1111/j.1399-3054.1986.tb03378.x
Yamunarani, K., Jaganathan, R., Bhaskaran, R., Govindaraju, P., and Velazhahan, R., Induction of early blight resistance in tomato by Quercus infectoria gall extract in association with accumulation of phenolics and defense-related enzymes, Acta Physiol. Plant., 2004, vol. 26, p. 281. https://doi.org/10.1007/S11738-004-0018-7
Johansen, T.J. and Mølmann, J.A.B., Seed potato performance after storage in light at elevated temperatures, Potato Res., 2018, vol. 61, p. 133. https://doi.org/10.1007/S11540-018-9363-6/FIGURES/3
Henrique, S.S.D., Zambolim, L., Rodrigues, F.A., Paul, P.A., Pádua, J.G., and Ribeiro, J.I., Field resistance of potato cultivars to foliar early blight and its relationship with foliage maturity and tuber skin types, Trop. Plant Path., 2014, vol. 39, p. 294.
Busnello, F.J., Boff, M.I.C., Agostinetto, L., Souza, Z. da S., and Boff, P., Potato genotypes reaction to early blight and late blight in organic cultivation, Ciência Rural, 2019, vol. 49. https://doi.org/10.1590/0103-8478CR20180469
Weber, B.N. and Jansky, S.H., Resistance to Alternaria solani in hybrids between a Solanum tuberosum haploid and S. raphanifolium, Phytopathology, 2012, vol. 102, p. 214. https://doi.org/10.1094/PHYTO-05-11-0146
Odintsova, T.I., Slezina, M.P., Istomina, E.A., Korostyleva, T.V., Kasianov, A.S., Kovtun, A.S., Makeev, V.J., Shcherbakova, L.A., and Kudryavtsev, A.M., Defensin-like peptides in wheat analyzed by whole-transcriptome sequencing: A focus on structural diversity and role in induced resistance, Peer J., 2019, vol. 2019, p. e6125. https://doi.org/10.7717/PEERJ.6125/SUPP-16
Toufiq, N., Tabassum, B., Bhatti, M.U., Khan, A., Tariq, M., Shahid, N., Nasir, I.A., and Husnain, T., Improved antifungal activity of barley derived chitinase I gene that overexpress a 32kDa recombinant chitinase in Escherichia coli host, Braz. J. Microbiol., 2018, vol. 49, p. 414. https://doi.org/10.1016/J.BJM.2017.05.007
Moravčíková, J., Matušíková, I., Libantová, J., Bauer, M., and Mlynárová, L., Expression of a cucumber class III chitinase and Nicotiana plumbaginifoliaclass I glucanase genes in transgenic potato plants, Plant Cell, Tissue Organ Cult., 2004, vol. 79, p. 161. https://doi.org/10.1007/S11240-004-0656-X
Islam, K.T., Velivelli, S.L.S., Berg, R.H., Oakley, B., and Shah, D.M., A novel bi-domain plant defensin MtD-ef5 with potent broad-spectrum antifungal activity binds to multiple phospholipids and forms oligomers, Sci. Rep., 2017, vol. 7, p. 16157. https://doi.org/10.1038/s41598-017-16508-w
Huang, X., Xie, W.J., and Gong, Z.Z., Characteristics and antifungal activity of a chitin binding protein from Ginkgo biloba, FEBS Letters, 2000, vol. 478, p. 123. https://doi.org/10.1016/S0014-5793(00)01834-2
Vasilchenko, A.S., Smirnov, A.N., Zavriev, S.K., Grishin, E.V., Vasilchenko, A.V., and Rogozhin, E.A., Novel thionins from black seed (Nigella sativa L.) demonstrate antimicrobial activity, Int. J. Pept. Res. Therap., 2017, vol. 23, p. 171. https://doi.org/10.1007/S10989-016-9549-1/FIGURES/5
Mithril, C. and Dragsted, L.O., Safety evaluation of some wild plants in the New Nordic Diet, Food Chem. Toxicol., 2012, vol. 50, p. 4461. https://doi.org/10.1016/J.FCT.2012.09.016
Yilmaz, S. and Ergün, S., Chickweed (Stellaria media) leaf meal as a feed ingredient for tilapia (Oreochromis mossambicus), J. Appl. Aquac., 2013, vol. 25, p. 329. https://doi.org/10.1080/10454438.2013.851531
Rogowska, M., Lenart, M., Srečec, S., Ziaja, M., Parzonko, A., and Bazylko, A., Chemical composition, antioxidative and enzyme inhibition activities of chickweed herb (Stelaria media L., Vill.) ethanolic and aqueous extracts, Ind. Crops Prod., 2017, vol. 97, p. 448. https://doi.org/10.1016/J.INDCROP.2016.12.058
Shukurov, R.R., Voblikova, V.D., Nikonorova, A.K., Komakhin, R.A., Komakhina, V.V., Egorov, T.A., Grishin, E.V., and Babakov, A.V., Transformation of tobacco and Arabidopsis plants with Stellaria media genes encoding novel hevein-like peptides increases their resistance to fungal pathogens, Transgenic Res., 2012, vol. 21, p. 313. https://doi.org/10.1007/s11248-011-9534-6
Vetchinkina, E.M., Komakhina, V.V., Vysotskii, D.A., Zaitsev, D.V., Smirnov, A.N., Babakov, A.V., and Komakhin, R.A., Expression of plant antimicrobial peptide pro-SmAMP2 gene increases resistance of transgenic potato plants to Alternaria and Fusarium pathogens, Russ. J. Genet., 2016, vol. 52, p. 939. https://doi.org/10.1134/s1022795416080147
Beliaev, D.V., Yuorieva, N.O., Tereshonok, D.V., Tashlieva, I.I., Derevyagina, M.K., Meleshin, A.A., Rogozhin, E.A., and Kozlov, S.A., High resistance of potato to early blight is achieved by expression of the Pro-SmAMP1 gene for hevein-like antimicrobial peptides from common chickweed (Stellaria media), Plants, 2021, vol. 10, p. 1395. https://doi.org/10.3390/PLANTS10071395
Muhammad, A.F., Naz, F., and Irshad, G., Important fungal diseases of potato and their management—a brief review, Mycopath., 2013, vol. 11, p. 45.
Timerbaev, V. and Dolgov, S., Functional characterization of a strong promoter of the early light-inducible protein gene from tomato, Planta, 2019, vol. 250, p. 1307. https://doi.org/10.1007/S00425-019-03227-X
Nagy, F., Boutry, M., Hsu, M.Y., Wong, M., and Chua, N.H., The 5′-proximal region of the wheat Cab-1 gene contains a 268-bp enhancer-like sequence for phytochrome response, EMBO J., 1987, vol. 6, p. 2537. https://doi.org/10.1002/J.1460-2075.1987.TB02541.X
An, G., Integrated regulation of the photosynthetic gene family from Arabidopsis thaliana in transformed tobacco cells, Mol. General Genet., 1987, vol. 207, p. 210. https://doi.org/10.1007/BF00331580
Bevan, M., Binary agrobacterium vectors for plant transformation, Nucl. Ac., Res., 1984, vol. 12, p. 8711. https://doi.org/10.1093/NAR/12.22.8711
Banerjee, A.K., Prat, S., and Hannapel, D.J., Efficient production of transgenic potato (S. tuberosum L. ssp. andigena) plants via Agrobacterium tumefaciens—mediated transformation, Plant Sci., 2006, vol. 170, p. 732. https://doi.org/10.1016/j.plantsci.2005.11.007
Lazo, G.R., Stein, P.A., and Ludwig, R.A., A DNA transformation–competent Arabidopsis genomic library in Agrobacterium, BioTechnology, 1991, vol. 9, p. 963. https://doi.org/10.1038/nbt1091-963
Deryabin, A.N. and Yur’eva, N.O., Formation and morphometric parameters of potato microtubers in vitro with different composition of sugars in the medium, Sel’skokhoz. Biol., 2011, vol. 1, p. 54. http://www.agrobiology.ru/1-2011deryabin-eng.html
Yuorieva, N.O., Voronkov, A.S., Tereshonok, D.V., Osipova, E.S., Platonova, E.V., and Belyaev, D.V., An assay for express screening of potato transformants by GFP fluorescence, Moscow Univ. Biol. Sci. Bull., 2018, vol. 73, p. 69. https://doi.org/10.3103/s0096392518020086
Nicot, N., Hausman, J.F., Hoffmann, L., and Evers, D., Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress, J. Exp. Bot., 2005, vol. 56, p. 2907. https://doi.org/10.1093/JXB/ERI285
Tzfira, T., Li, J., Lacroix, B., and Citovsky, V., Agrobacterium T-DNA integration: molecules and models, Trends Genet., 2004, vol. 20, p. 375. https://doi.org/10.1016/J.TIG.2004.06.004
Cluster, P.D., O’Dell, M., Metzlaff, M., and Flavell, R.B., Details of T-DNA structural organization from a transgenic Petunia population exhibiting co-suppression, Plant Mol. Biol., 1996, vol. 32, p. 1197. https://doi.org/10.1007/BF00041406
Escoubas, J.M., Lomas, M., LaRoche, J., and Falkowski, P.G., Light intensity regulation of cab gene transcription is signaled by the redox state of the plastoquinone pool, Proc. Natl. Acad. Sci., 1995, vol. 92, p. 10237. https://doi.org/10.1073/PNAS.92.22.10237
Czajka, K.M. and Nkongolo, K., Transcriptome analysis of trembling aspen (Populus tremuloides) under nickel stress, PLOS ONE, 2022, vol. 17, p. e0274740. https://doi.org/10.1371/JOURNAL.PONE.0274740
Daley, M., Knauf, V.C., Summerfelt, K.R., and Turner, J.C., Co-transformation with one Agrobacterium tumefaciens strain containing two binary plasmids as a method for producing marker-free transgenic plants, Plant Cell Rep., 1998, vol. 17, p. 489. https://doi.org/10.1007/S002990050430
Funding
The research was carried out within the state assignment of Ministry of Science and Higher Education of the Russian Federation (theme No. 122042600086-7).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving humans and animals as research subjects.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Additional information
Translated by M. Shulskaya
Rights and permissions
About this article
Cite this article
Beliaev, D.V., Yourieva, N.O., Tereshonok, D.V. et al. Early Blight Resistance of Transgenic Potato Plants Expressing the ProSmAMP1 Gene for Antimicrobial Peptides under the Control of a Light-Inducible Cab Promoter. Russ J Plant Physiol 70, 57 (2023). https://doi.org/10.1134/S1021443722700042
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S1021443722700042