Abstract
The tomato Solanum lycopersicum L. is a convenient model for studying carotenoid metabolism due to the wide variety of carotenoid-mediated pigmentation of the ripe fruit. Carotene cis-trans isomerase CRTISO catalyzes cis-trans isomerization of double bonds leading to the formation of all-trans-lycopene, which is the most powerful antioxidant among carotenoids and a substrate for subsequent synthesis of α-, β-carotenes, and xanthophylls. In this work, 18 accessions of tomato cultivars and lines differing in the color of ripe fruit have been analyzed. The obtained biochemical data showed a dependence of fruit color on the content and composition of carotenoids and the presence or absence of chlorophylls. Expression analysis of three CRTISO homologous genes performed in silico has shown that the highest expression level in the fruit is only characteristic of gene CRTISO that has the maximal transcription at the stages of color change (from green to red) and biological ripeness of the fruit. Quantitative real-time PCR did not reveal any strong correlation between the level of CRTISO gene expression and total carotenoids, which may be explained by a different quantity of metabolites preceding prolycopene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
A large class of secondary metabolites (carotenoids) is produced by plants, bacteria, and fungi, whereas animals (including humans) obtain them only with food [1]. Carotenoids are an important component ensuring protection of plants' photosynthetic apparatus from photooxidative damage caused by an excess of light. Moreover, oxidative breakdown of carotenoids may produce signal compounds, such as β-cyclocitral, which is involved in regulating the expression of nuclear genes in response to photooxidative stress, whereas their enzymatic conversions result in the formation of hormones (abscisic acid and strigolactones) [2]. At the center of carotenoid molecule is a chromophore—a system of conjugated double bonds; each bond may exist in a cis- or trans- configuration and, thereby, govern the wavelength and intensity of the absorbed light [3].
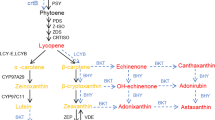
In fruit and vegetable crops, carotenoids also act as pigments that accumulate in chromoplasts and account for red, orange, and yellow color of flowers and fruits [2, 4]. Among vegetable crops, one of the main sources of carotenoids is the tomato Solanum lycopersicum L., the fruits of which are mostly red in color due to the accumulation of all-trans-lycopene. This compound is synthesized from the precursor of all carotenoids (colorless 15-cis-phytoene) in several steps, including two desaturations and two cis-trans isomerizations [3]. Carotenoid isomerization during desaturation is a significant difference between carotenoid biosynthesis in plants and bacteria; in bacteria, molecules from phytoene to lycopene are all-trans-isomers. These reactions are performed by two desaturases (phytoene desaturase PDS and ζ-carotene desaturase ZDS) and two isomerases (ζ-carotene isomerase Z-ISO and carotene cis-trans isomerase CRTISO) (Fig. 1а) [3]. Orthologs of isomerases are present only in cyanobacteria and plants, while in purple photosynthetic bacteria and non-photosynthetic organisms, the functions of desaturation and isomerization are combined in one enzyme of the carotene desaturase type CRTI [3]. CRTISO is considered an evolutionary derivative of CRTI and catalyzes cis-trans isomerization of double bonds, leading to the formation of all-trans-lycopene—a substrate for subsequent cyclization with the formation of all-trans-α-β-carotenes controlled by the corresponding lycopene cyclases [5].
Scheme of carotenoid biosynthesis in tomato fruit (a) and photographs of ripe fruit of tomato accessions studied in this work: (b) L-Nesozrevayushchii, (c) L-Cherry Limonno-Zheltye, (d) cv. Viking, (e) L-Zolotoi Lotos, (f) cv. Grot, (g) cv. Blagodatnyi, (h) cv. Lotos, (i) cv. Podarok k Yubileyu, (j) cv. Malinovyi Silach, (k) cv. Gayana, (l) cv. Garmoshka, (m) cv. Russkii Razmer, (n) cv. Dubok, (o) L-411, (p) L-Cherry Rozovye, (q) L-Cherry Arbuz, (r) L-176, (s) L-Cherry Korichnevo-Fioletovye. GGPP—geranylgeranyl pyrophosphate; PSY1—phytoene synthase 1; PDS—phytoene desaturase; Z-ISO—ζ-carotene isomerase; ZDS—ζ-carotene desaturase; CRTISO—carotene cis-trans-isomerase.
Isomerase gene CRTISO was cloned for the first time in Arabidopsis thaliana as a result of analysis of the ccr2 mutation, which cause an accumulation of acyclic isomers of carotene in the etioplasts and a decrease in lutein content in the leaf chloroplasts [6]. At the same time, the CRTISO gene was detected in the tomato S. lycopersicum, mutant tangerine, the fruits and flowers of which were orange in color, in contrast to the red (fruit) and yellow (flower) in wild type tomato [7]. Differences in fruit color were explained by the different composition of accumulated carotenoids: prolycopene in the tangerine mutant, and all-trans-lycopene in the wild type tomato [7]. Gene CRTISO accounting for the tangerine phenotype is located on chromosome 10; it is one of three genes in the tomato genome (the other two genes are CRTISO-L1 and CRTISO-L2) encoding carotenoid isomerases, which are enzymes of the redox type, structurally related to bacterial-type phytoene desaturase CRTI [8]. Two mutant tangerine alleles were studied, the loss of function of which was associated with a deletion of 282 bp (24 bp in exon I and 258 bp in intron I) in the CRTISO genomic sequence (allele 1) and impaired gene expression due to deletion of 348 bp in the promoter (allele 2). In the case of an exon-intron deletion, the splicing site is removed and the resulting mRNA contains an early stop codon that interrupts the synthesis of the functional CRTISO protein [7].
It was shown that CRTISO is expressed in all tissues of wild type tomato, and its expression considerably increases during fruit ripening (with a peak in ripe fruit) and in flowers [7]. It is interesting that green tissues do not have rigorous requirements for the presence of CRTISO activity. Thus, developing leaves of the tangerine mutant are prone to discoloration; however, mature leaves are phenotypically unaffected and in the light accumulate almost the same carotenoids as the wild type tomato, while in the dark, the biosynthesis of carotenoids does not proceed further than prolycopene [7]. This suggested that the CRTISO activity is necessary for cis-trans isomerization in the absence of illumination, and in the light, it is successfully replaced by photoisomerization [5, 7]. It was also shown that tomato CRTISO is able to isomerize adjacent cis-double bonds at C7 and C9 pairwise to the trans-configuration, but not single cis-double bonds at positions C9 and C9' [9].
Subsequently, the carotene isomerase CRTISO was examined in other species, mostly crops. For example, the role of CRTISO in maintaining the photosynthetic release of oxygen in plants was shown using mutants of rice Oryza sativa [10]. In Brassica rapa, based on polymorphisms in the CRTISO homologs (Bra031539; BrCRTISO1), molecular markers associated with the orange color of the head were developed [11, 12]. It was shown that concentrations of chlorophyll and carotenoids related to the color of Chinese kale may be altered through CRISPR/Cas9 targeted editing of the carotene isomerase gene BoaCRTISO [13]. Four CRTISO homologous genes were identified and functionally characterized in the orange Citrus sinensis, and a recovery of carotenoid biosynthesis was shown in tomato tangerine mutant plants with overexpression of CsCRTISO [14]. Data on the CRTISO gene were used to clarify the history of carrot domestication [15]. In addition, CRTISO was shown to be involved in the response to stresses, for example, in carrot plant’s response to root infection with the obligate parasite Phelipanche aegyptiaca [16]. Overexpression of CRTISO significantly increases salt resistance and the level of carotenoid accumulation in transgenic tobacco plants [17].
This study is focused on the evaluation of a possible correlation between the CRTISO gene expression profile in ripe fruit of tomato S. lycopersicum cultivars, differing in color and the content and composition of carotenoids.
MATERIALS AND METHODS
We used plant material of ten cultivars and eight breeding lines of tomato (S. lycopersicum), differing in ripe fruit color (Figs. 1b–1s, Table S1). The plants were grown in 2021 until fruiting in a film greenhouse at the Federal Research Vegetable Center (FRVC, Moscow region, Russia) and then kept at the experimental climate control facility (Institute of Bioengineering, Research Center of Biotechnology, Russian Academy of Sciences).
In September 2021, ripe fruits (final stage of ripening; Red Ripe, RR) of each cultivar (one fruit each from two plants) were picked, ground (peel and pulp together) in liquid nitrogen, and used for expression and biochemical studies.
To analyze gene expression, total RNA was isolated from 50‒100 mg of ripe fruit tissue (RNeasy Plant Mini Kit; Qiagen, Germany), purified from DNA (RNase free DNasy set; Qiagen, Germany) and used for cDNA synthesis (GoScriptтм Reverse Transcription System; Promega, United States) according to the manufacturer’s protocols. Concentrations of RNA and cDNA were determined using a Qubit 4 fluorometer (Thermo Fisher Scientific, United States) with appropriate reagents (Qubit RNA HS Assay Kit and Qubit DS DNA HS Assay Kit; Invitrogen, United States). RNA quality was assessed by electrophoresis in 1.5% agarose gel.
Gene-specific primers (forward 5'-ATGAAGCGAAGAAAGAGGTTGT-3'; reverse 5'-GCAAGGTATCGTCTGTGGGTCT-3') for expression analysis were developed based on the tomato CRTISO sequence available at GenBank NCBI (chromosome 10, Solyc10g081650, https://www.solgenomics.net/; Gene ID: 101267857; https://www.ncbi.nlm.nih.gov/) [18]. According to the results of the comparative analysis, these primers do not anneal on the sequences of two other CRTISO homologous genes: CRTISO-L1 (chromosome 5, Solyc05g010180.2; Gene ID: 101247017) and CRTISO-L2 (chromosome 2, Gene ID: 101259799; or chromosome 7, Solyc07g021640). Primers were synthesized at ZAO Evrogen (Moscow, Russia). Real-time quantitative PCR (RT-qPCR) was performed using a CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, United States) in two biological and three technical replicates (Reaction mixture for RT-qPCR in the presence of SYBR Green I and ROX, ZAO Syntol, Moscow, Russia). 100 ng of the cDNA template were used for the reaction conducted under following conditions: 95°C for 5 min; 40 cycles (95°C for 15 s, 60°C for 40 s). To normalize the expression, tomato reference genes Expressed and ACTIN2 were used [19]. The obtained results were statistically processed (Graph Pad Prism v. 8; GraphPad Software Inc., San Diego, CA, United States; https://www. graphpad.com/scientific-software/prism/). The data were expressed as the mean ± standard deviation (SD) based on two biological and three technical replicates for each cDNA variant. Differences in gene expression were assessed using unequal variance (Welch’s) t-test (P < 0.05 points to statistical significance of the difference). Linear regressions of CRTISO gene expression and the content of carotenoids, as well as multiple correlation coefficients (R2), were calculated using GraphPad Prism v. 8.
Expression analysis of CRTISO homologous genes was performed in silico using TomExpress data (http://tomexpress.toulouse.inra.fr/).
Biochemical assay of the content (mg/g of fresh weight) of chlorophylls (a and b), lycopene, β-carotene, and the total amount of carotenoids (x + c; carotenes + xanthophylls as well as prolycopene and lycopene) in the ripe fruit of all analyzed tomato accessions was performed according to a modified Folch protocol [19, 20] in two biological and three technical replicates. Absorption spectra were recorded using an Eppendorf BioSpectrometer® basic (Eppendorf, Germany) and a Cary 50 (Agilent Technology, United States) spectrophotometers.
An additional detailing of the content of carotenoids in the ripe fruit of tomato accessions was performed using the Shimadzu HPLC (high-performance liquid chromatography) system (Shimadzu, Kyoto, Japan) at 22°C as described previously [21, 22]. The HPLC system comprised an LC-10ADVP pump with an FCV-10ALVP module, an SPD-M20A diode array detector, and a CTO-20AC thermostat. Separation was performed on an Agilent Zorbax SB-C18 reversed phase column (5 µm, 4.6 × 250 mm) (Agilent, Santa Clara, United States). Solvent flow rate was 1 mL/min. Carotenoids were identified by their retention time and absorption spectra. Quantification of each carotenoid was performed by comparing its peak area in the spectral region of 270‒800 nm with the sum of all carotenoid peaks, taken as 100%; calculations were performed with LC-solution software (Shimadzu, Kyoto, Japan) using molar extinction coefficients [23]. In four tomato accessions analyzed, the total carotenoid content (x + c) was additionally measured in 100% petroleum ether according to Rodriguez-Amaya [24]. Concentration of each carotenoid (µg/g fr wt) was calculated by comparing values of total carotenoids with a percentage (mol %) of individual types of carotenoids.
RESULTS
Pigmentation of a Ripe Tomato Fruit Depends on the Content and Composition of Carotenoids
Plant material (cultivars and breeding lines) was chosen by differences in the color of the ripe fruit (Figs. 1b–1s). Thus, ripe fruits of the line L-Nesozrevayushchii were pale green. In cv. Viking and two lines (L-Cherry Limonno-Zheltye and L-Zolotoi Lotos), fruits showed shades of yellow and orange. In nine cultivars (Grot, Blagodatnyi, Lotos, Podarok k Yubileyu, Malinovyi Silach, Gayana, Garmoshka, Russkii Razmer, and Dubok) and two lines (L-Cherry Rozovye and L-411), fruit color varied from pink and crimson to red. Fruits of the three lines (L-Cherry Arbuz, L-Cherry Korichnevo-Fioletovye, and L-176) were reddish brown with purple shade or patches.
To assess the dependence of tomato fruit color on the content of carotenoids, we determined the content of lycopene, β-carotene, total carotenoids (x + c), and chlorophylls (a and b) in ripe fruit (peel and pulp together) in all 18 accessions (Table S1). According to the obtained data, the highest content of total carotenoids (0.1518 mg/g fr wt) was detected in the line L-Cherry Korichnevo-Fioletovye, while the lowest (0.0102 mg/g fr wt) was in the yellow-fruited line L-Cherry Limonno-Zheltye (Table S1, Fig. 2c). At the same time, the content of lycopene or β-carotene did not strongly correlate with fruit color. Pale green and yellow orange fruits accumulated traces of lycopene (L-Zolotoi Lotos) or entirely lacked it, whereas red or brown purple fruits were characterized by a relatively high content of lycopene: from 0.0196 mg/g fr wt (cv. Dubok) to 0.1153 mg/g fr wt (L-Cherry Arbuz) (Table S1 and Fig. 2b). Concentration of β-carotene had trace values in three cultivars (Viking, Garmoshka, and Podarok k Yubileyu) and three lines (L-411, L‑Cherry Limonno-Zheltye, and L-Cherry Arbuz). The highest content of β-carotene was detected in ripe fruit of the lines L-Nesozrevayushchii (0.0358 mg/g fr wt), L‑Cherry Korichnevo-Fioletovye (0.0345 mg/g fr wt), and L-Zolotoi Lotos (0.0237 mg/g fr wt); the remaining accessions accumulated identical amount of β-carotene (0.0121–0.0185 mg/g fr wt) (Table S1, Fig. 2a).
Content of (a) β-carotene, (b) lycopene, and (c) total carotenoids in ripe fruit of the studied tomato accessions: (1) L-Nesozrevayushchii, (2) L-Cherry Limonno-Zheltye, (3) cv. Viking, (4) L-Zolotoi Lotos, (5) cv. Grot, (6) cv. Blagodatnyi, (7) cv. Lotos, (8) cv. Podarok k Yubileyu, (9) cv. Malinovyi Silach, (10) cv. Gayana, (11) cv. Garmoshka, (12) cv. Russkii Razmer, (13) cv. Dubok, (14) L-411, (15) L-Cherry Rozovye, (16) L-Cherry Arbuz, (17) L-176, (18) L-Cherry Korichnevo-Fioletovye. Colored rectangles roughly indicate the color of the ripe fruit. Concentration (mg/g fr wt) is shown as a mean ± standard error based on two biological and three technical replicates.
Chlorophylls (a + b) were found only in the fruits of the line L-Nesozrevayushchii in a quantity similar to the concentration of β-carotene (Table 1).
To clarify possible correlations between ripe fruit color and carotenoid composition, as well as the extent of conversion of precursors to all-trans-lycopene and downstream carotenoids, an additional analysis was carried out. The spectrum of carotenoids was detailed by HPLC in four tomato accessions, contrasting in the color of the ripe fruit: the green-fruited line L-Nesozrevayushchii, the yellow-fruited cv. Viking, the red-fruited cv. Malinovyi Silach, and the brown-red-fruited line L-176. Prolycopene was absent in all samples. In both red-fruited accessions, colorless carotenoid precursors (phytoene and phytofluene; 0.0164–0.0202 mg/g fr wt, ~23–31% of total carotenoids) and derivatives of ζ-carotene (traces) were detected (Table 1). In the yellow-orange fruits of the cv. Viking, carotenoids included significant amounts of lutein derivatives. Except for L-Nesozrevayushchii, the fruits of the examined accessions contained β-cryptoxanthin and (all-E)-lutein (Table 1). Red pigment lycopene was not detected in the fruits of the cv. Viking and the line L-Nesozrevayushchii, while it was a predominant carotenoid in the fruits of the cv. Malinovyi Silach (40%) and the line L-176 (37%). Orange β-carotene prevailed in the fruits of the line L-Nesozrevayushchii (100%) and the cv. Viking (63%); however, its content in red-fruited accessions was 25% (cv. Malinovyi Silach) and 33% (L-176).
Expression of CRTISO Does Not Correlate with Total Carotenoids in a Ripe Tomato Fruit
It is known that tomato genome carries three genes encoding carotene isomerases (CRTISO, CRTISO-L1, and CRTISO-L2). To find out which of them is active in tomato fruit, we made a preliminary in silico analysis (TomExpress) of these genes’ expression (Fig. 3). It was shown that the CRTISO gene is expressed in all examined tissues and organs with a peak in the fruit, as well as in the leaf and flower. In the fruit, the highest level of CRTISO transcription was observed at the stage of color change from green to red (breaker, BR) and at biological ripeness (red ripe, RR) (Fig. 3). Transcription of another CRTISO-L1 gene is most active in the tomato flower; its expression is much lower than that of the CRTISO, and it is almost zero in the fruit. mRNA of the third gene CRTISO-L2 was not detected in the examined tissues (Fig. 3). Considering these data, we used only the CRTISO gene in further analysis.
Relative transcription level of CRTISO (Solyc10g081650), CRTISO-L1 (Solyc05g010180.2), and CRTISO-L2 (Solyc07g021640) genes in different organs of tomato Solanum lycopersicum according to TomExpress (http://tomexpress.toulouse.inra.fr/login). DPA—days post anthesis (open flower); DPG—days post germination; BR, ORG—stage of fruit color change; RED—biological ripeness of the fruit; MG—mature green fruit of final size; IG—immature green fruit.
To verify the assumption of a correlation between the quantity of all-trans-lycopene or the total carotenoids with the activity of the CRTISO, we analyzed CRTISO expression in ripe fruits of 18 studied tomato accessions. The highest transcription level was observed in cv. Viking and the lowest was in L-Nesozrevayushchii (Fig. 4a). In most red-fruited cultivars, the gene expression reliably did not differ except for cv. Gayana and line L-Cherry Rozovye (with the lowest transcription among red-fruited accessions), as well as cv. Malinovyi Silach and cv. Garmoshka (the highest transcription level among red-fruited accessions) (Fig. 4a).
(a) Relative CRTISO transcription level in ripe fruit of analyzed tomato accessions: (1) L-Nesozrevayushchii, (2) L-Cherry Limonno-Zheltye, (3) cv. Viking, (4) L-Zolotoi Lotos, (5) cv. Grot, (6) cv. Blagodatnyi, (7) cv. Lotos, (8) cv. Podarok k Yubileyu, (9) cv. Malinovyi Silach, (10) cv. Gayana, (11) cv. Garmoshka, (12) cv. Russkii Razmer, (13) cv. Dubok, (14) L-411, (15) L-Cherry Rozovye, (16) L-Cherry Arbuz, (17) L-176, (18) L-Cherry Korichnevo-Fioletovye. Colored rectangles roughly indicate the color of the ripe fruit. Letters (a, b, c, d, e, f) show statistically significant differences (P < 0.05). (b) Estimation of the correlation between the level of CRTISO expression and the total content of carotenoids (numbering of accessions: (1) cv. Viking, (2) cv. Grot, (3) cv. Blagodatnyi, (4) cv. Lotos, (5) cv. Malinovyi Silach, (6) cv. Garmoshka, (7) cv. Russkii Razmer, (8) cv. Gayana, (9) cv. Podarok k Yubileyu, (10) cv. Dubok, (11) L-Nesozrevayushchii, (12) L-Cherry Limonno-Zheltye, (13) L-Zolotoi Lotos, (14) L-Cherry Rozovye, (15) L-Cherry Arbuz, (16) L-Cherry Korichnevo-Fioletovye, (17) L-411, (16) L-Cherry Arbuz, (18) L-176). All samples were taken into account; coefficient R2 = 0.03248; (c) five samples (L-Nesozrevayushchii, L-Cherry Limonno-Zheltye, cvs. Viking, Gayana, and Garmoshka) were excluded; R2 = 0.3383.
An assessment of the dependence of the content of total carotenoids on the level of CRTISO expression showed no correlation. Thus, the coefficient of determination R2 between the content of total carotenoids and the level of CRTISO expression in all analyzed samples was 0.03248 (Fig. 4b), while R2 without five samples that drop out of the previous graph (L-Nesozrevayushchii, L-Cherry Limonno-Zheltye, cv. Viking, cv. Gayana, and cv. Garmoshka) was 0.3383 (Fig. 4c). Both results do not meet the correlation criterion (R2 ≥ 0.7).
DISCUSSION
Tomato (S. lycopersicum) fruits are an important dietary source of lycopene and other carotenoids, the consumption of which is associated with a reduced risk of oncology, cardiovascular diseases and death-rate [25]. In the course of ripening, the identity of cell plastids in the fruit tissues changes: chloroplasts containing chlorophyll turn into chromoplasts, certain structures of which can accumulate carotenoids. As a result, depending on the content and ratio of types of carotenoids and residual chlorophyll, the green fruit acquires a yellow, orange, or red color (a variety of shades with characteristic absorption spectra) [25, 27]. A wide diversity of fruit color makes tomato S. lycopersicum an appropriate model for studying the biosynthesis and accumulation of carotenoids in fleshy fruit.
Our study was focused on determining the content and composition of carotenoids in the ripe fruit of tomato accessions and their possible dependence on the expression of the carotene isomerase gene CRTISO. This enzyme is responsible for cis-trans isomerization of prolycopene and, thus, determines the total content of carotenoids: all-trans-lycopene (red pigment) and downstream yellow and orange compounds (α-, β-carotenes and xanthophylls). In this work, we used plant material of tomato with different color of ripe fruit, as well as tomato accession of the line L-Nesozrevayushchii, the fruit of which essentially does not change its green color when ripe.
The obtained biochemical data (Tables S1, 1; Fig. 2) confirmed the dependence of fruit color (Fig. 1) on the content and composition of pigments. Thus, the yellow-orange color of cv. Viking fruit may be due to the absence of lycopene and the presence of β-carotene and luteins. The fruit color in cv. Malinovyi Silach and line L-176 can be explained by the presence of much lycopene and β-carotene. At the same time, the deep crimson-red fruit color in cv. Malinovyi Silach may link to almost a twofold predominance of lycopene (red) over β-carotene (orange), while brown-red fruit color in L-176 can be due to approximately equal content of lycopene and β-carotene and the presence of a significant amount of (all-E)-lutein (yellow). Finally, pale green fruit color with yellow shade in L-Nesozrevayushchii is accounted for by equal quantities of β-carotene and chlorophylls.
Line L-Nesozrevayushchii was previously obtained by crossing the cv. Zemba (VIR, St. Petersburg, Russia) and the line LA1795 homozygous by rin mutation (varietal background is unknown; TGRC, Davis, California, United States) [28]. Line LA1795 produces ripe fruit with signs of unripeness (green color, no accumulation of moisture as it ripens, and firm texture lasting longer than in cultivars). This is due to the suppression of cell respiration and ethylene release that prevents the biosynthesis and accumulation of carotenoids and aromatics, as well as fruit softening [29]. rin mutation is caused by an extended deletion in the sequence of chromosome 5, leading to the fusion of adjacent truncated genes RIPENING INHIBITOR (RIN) and MACROCALYX (MC) (locus RIN-MC) [29, 30]. The transcription factor RIN is considered to be the main regulator of tomato fruit ripening; it governs transcription of numerous genes responsible for all the stages of fruit ripening, including structural genes for biosynthesis of carotenoids, CRTISO in particular [18, 30‒33].
Considering that CRTISO is a RIN target, it was expected that its expression in ripe fruit of L-Nesozrevayushchii would be considerably reduced. The results of expression analysis confirmed the expectations: the level of CRTISO transcription in this line (genotype rin/RIN) turned out to be the lowest as compared with other examined accessions (genotype RIN/RIN) (Fig. 4a).
It is interesting that the fruit of all four examined accessions (cv. Viking, cv. Malinovyi Silach, and lines L-Nesozrevayushchii and L-176) entirely lacked prolycopene (7,9,9',7'-tetra-cis-lycopene, precursor to all-trans-lycopene), which points to its full isomerization to all-trans-lycopene by CRTISO (Table 2). Previously, it was shown that silencing of gene CRTISO resulted in prolycopene accumulation and all-trans-lycopene deficiency in tomato fruit [34]. This suggested a correlation between the level of CRTISO expression and the total carotenoid content (lycopene + downstream carotenoids, except for ζ-carotene derivatives). However, in our study, no clear dependence was observed on a wide range of red-fruited cultivars (Figs. 4b, 4c). Also, the data obtained for the yellow-fruited cv. Viking and line L-Nesozrevayushchii (respectively: the highest and lowest levels of CRTISO expression; minimal and similar to other samples content of total carotenoids) did not fit into the correlation (Table 1, Fig. 4a). This may be accounted for by a total lack of any carotenoid precursors in the fruits of these two accessions in contrast to cv. Malinovyi Silach and line L-176, which fruits accumulated 32 and 23% of such precursors, respectively (Table 1). It can be assumed that in the fruits of cv. Viking, the carotene isomerase CRTISO, at the present level of expression, would successfully modify a significantly larger amount of prolycopene. At the same time, in the fruits of the line L-Nesozrevayushchii, a moderate CRTISO expression was sufficient for the isomerization of the entire substrate.
Thus, the obtained data show the dependence of the color of tomato fruit on the content and composition of carotenoids and the presence of chlorophylls. The absence of a pronounced correlation between the level of CRTISO expression and the total carotenoid amount may be explained by a different content of prolycopene precursors, which is determined by the activity, first of all, of phytoene synthase PSY1 and, then, of desaturases (PDS and ZDS) and ζ-carotene isomerase Z-ISO. Therefore, the assessment of the correlation between the level of CRTISO transcription and the total carotenoids is possible when prolycopene is detected. In addition, the production of end metabolites og the carotenoid pathway (hormones) and the possible degradation of carotenoids should be taken into account. Moreover, the amount of various carotenoids depends on the activity of the pathway enzymes rather than on the level of transcriptional activity of genes encoding these enzymes.
Change history
21 September 2022
An Erratum to this paper has been published: https://doi.org/10.1134/S1021443722330019
REFERENCES
Langi, P., Kiokias, S., Varzakas, T., and Proestos, C., Carotenoids: from plants to food and feed industries, Methods Mol. Biol., 2018, vol. 1852, p. 57. https://doi.org/10.1007/978-1-4939-8742-9_3
Llorente, B., Martinez-Garcia, J.F., Stange, C., and Rodriguez-Concepcion, M., Illuminating colors: regulation of carotenoid biosynthesis and accumulation by light, Curr. Opin. Plant Biol., 2017, vol. 37, p. 49. https://doi.org/10.1016/j.pbi.2017.03.011
Giuliano, G., Giliberto, L., and Rosati, C., Carotenoid isomerase: a tale of light and isomers, Trends Plant Sci., 2002, vol. 7, p. 427. https://doi.org/10.1016/s1360-1385(02)02329-4
Lado, J., Zacarías, L., and Rodrigo, M.J., Regulation of carotenoid biosynthesis during fruit development, in Carotenoids in Nature: Biosynthesis, Regulation and Function, Subcell. Biochem. Ser., vol. 79, Cham: Springer-Verlag, 2016, p. 161. https://doi.org/10.1007/978-3-319-39126-7_6
Yu, Q., Ghisla, S., Hirschberg, J., Mann, V., and Beyer, P., Plant carotene cis-trans isomerase CRTISO: a new member of the FADRED-dependent flavoproteins catalyzing non-redox reactions, J. Biol. Chem., 2011, vol. 286, p. 8666. https://doi.org/10.1074/jbc.M110.208017
Park, H., Kreunen, S.S., Cuttriss, A.J., DellaPenna, D., and Pogson, B.J., Identification of the carotenoid isomerase provides insight into carotenoid biosynthesis, prolamellar body formation, and photomorphogenesis, Plant Cell, 2002, vol. 14, p. 321. https://doi.org/10.1105/tpc.010302
Isaacson, T., Ronen, G., Zamir, D., and Hirschberg, J., Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of β-carotene and xanthophylls in plants, Plant Cell, 2002, vol. 14, p. 333. https://doi.org/10.1105/tpc.010303
Tomato Genome Consortium, The tomato genome sequence provides insights into fleshy fruit evolution, Nature, 2012, vol. 485, p. 635. https://doi.org/10.1038/nature11119
Isaacson, T., Ohad, I., Beyer, P., and Hirschberg, J., Analysis in vitro of the enzyme CRTISO establishes a poly-cis-carotenoid biosynthesis pathway in plants, Plant Physiol., 2004, vol. 136, p. 4246. https://doi.org/10.1104/pp.104.052092
Wei, J., Xu, M., Zhang, D., and Mi, H., The role of carotenoid isomerase in maintenance of photosynthetic oxygen evolution in rice plant, Acta Biochim. Biophys. Sin., 2010, vol. 42, p. 457. https://doi.org/10.1093/abbs/gmq044
Zou, C.L., Zheng, Y., Wang, P., Zhang, X., Wang, Y.H., Liu, Z.Y., and Feng, H., Fine mapping and characterization of the or gene in Chinese cabbage (Brassica rapa L. ssp. pekinensis), Genet. Mol. Res., 2016, vol. 15, p. gmr8370. https://doi.org/10.4238/gmr.15028370
Lee, S., Lee, S.C., Byun, D.H., Lee, D.Y., Park, J.Y., Lee, J.H., Lee, H.O., Sung, S.H., and Yang, T.J., Association of molecular markers derived from the BrCRTISO1 gene with prolycopene-enriched orange-colored leaves in Brassica rapa, Theor. Appl. Genet., 2014, vol. 127, p. 179. https://doi.org/10.1007/s00122-013-2209-3
Sun, B., Jiang, M., Zheng, H., Jian, Y., Huang, W.L., Yuan, Q., Zheng, A.H., Chen, Q., Zhang, Y.T., Lin, Y.X., Wang, Y., Wang, X.R., Wang, Q.M., Zhang, F., and Tang, H.R., Color-related chlorophyll and carotenoid concentrations of Chinese kale can be altered through CRISPR/Cas9 targeted editing of the carotenoid isomerase gene BoaCRTISO, Hortic. Res., 2020, vol. 7, p. 161. https://doi.org/10.1038/s41438-020-00379-w
Pinheiro, T.T., Peres, L.E.P., Purgatto, E., Latado, R.R., Maniero, R.A., Martins, M.M., and Figueira, A., Citrus carotenoid isomerase gene characterization by complementation of the “Micro-Tom” tangerine mutant, Plant Cell Rep., 2019, vol. 38, p. 623. https://doi.org/10.1007/s00299-019-02393-2
Soufflet-Freslon, V., Jourdan, M., Clotault, J., Huet, S., Briard, M., Peltier, D., and Geoffriau, E., Functional gene polymorphism to reveal species history: the case of the CRTISO gene in cultivated carrots, PLoS One, 2013, vol. 8, p. e70801. https://doi.org/10.1371/journal.pone.0070801
Emran, S., Nawade, B., Yahyaa, M., Abu Nassar, J., Tholl, D., Eizenberg, H., and Ibdah, M., Broomrape infestation in carrot (Daucus carota): changes in carotenoid gene expression and carotenoid accumulation in the parasitic weed Phelipanche aegyptiaca and its host, Sci. Rep., 2020, vol. 10, p. 324. https://doi.org/10.1038/s41598-019-57298-7
Li, C., Ji, J., Wang, G., Li, Z., Wang, Y., and Fan, Y., Over-expression of LcPDS, LcZDS, and LcCRTISO, genes from wolfberry for carotenoid biosynthesis, enhanced carotenoid accumulation, and salt tolerance in tobacco, Front. Plant Sci., 2020, vol. 11, p. 119. https://doi.org/10.3389/fpls.2020.00119
Li, S., Xu, H., Ju, Z., Cao, D., Zhu, H., Fu, D., Grierson, D., Qin, G., Luo, Y., and Zhu, B., The RIN-MC fusion of MADS-box transcription factors has transcriptional activity and modulates expression of many ripening genes, Plant Physiol., 2018, vol. 176, p. 891. https://doi.org/10.1104/pp.17.01449
Efremov, G.I., Slugina, M.A., Shchennikova, A.V. and Kochieva, E.Z., Differential regulation of phytoene synthase PSY1 during fruit carotenogenesis in cultivated and wild tomato species (Solanum section Lycopersicon), Plants, 2020, vol. 9, p. 1169. https://doi.org/10.3390/plants9091169
Filyushin, M.A., Dzhos, E.A., Shchennikova, A.V., and Kochieva, E.Z., Dependence of pepper fruit color on basic pigments ratio and expression pattern of carotenoid and anthocyanin biosynthesis genes, Russ. J. Plant Physiol., 2020, vol. 67, p. 1054. https://doi.org/10.1134/S1021443720050040
Ashikhmin, A., Makhneva, Z., Bolshakov, M., and Moskalenko, A., Incorporation of spheroidene and spheroidenone into light-harvesting complexes from purple sulfur bacteria, J. Photochem. Photobiol. B, 2017, vol. 170, p. 99. https://doi.org/10.1016/j.jphotobiol.2017.03.020
Pashkovskiy, P., Kreslavski, V., Khudyakova, A., Ashikhmin, A., Bolshakov, M., Kozhevnikova, A., Kosobryukhov, A., Kuznetsov, V.V., and Allakhverdiev, S.I., Effect of high-intensity light on the photosynthetic activity, pigment content and expression of light-dependent genes of photomorphogenetic Solanum lycopersicum hp mutants, Plant Physiol. Biochem., 2021, vol. 167, p. 91. https://doi.org/10.1016/j.plaphy.2021.07.033
Britton, G., UV/visible spectroscopy, in The Carotenoid Series, Vol. 1B: Spectroscopy, Britton G., Liaaen-Jensen S., and Pfander H., Eds., Basel: Birkhäuser Verlag, 1995.
Rodriguez-Amaya, D.B., A Guide to Carotenoid Analysis in Foods, Washington, DC: Int. Life Sci. Inst., 2001, p. 45. https://pdf.usaid.gov/pdf_docs/pnacq929.pdf.
Aune, D., Keum, N., Giovannucci, E., Fadnes, L.T., Boffetta, P., Greenwood, D.C., Tonstad, S., Vatten, L.J., Riboli, E., and Norat, T., Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: a systematic review and dose-response meta-analysis of prospective studies, Am. J. Clin. Nutr., 2018, vol. 108, p. 1069. https://doi.org/10.1093/ajcn/nqy097
Yoo, H.J., Park, W.J., Lee, G.M., Oh, C.S., Yeam, I., Won, D.C., Kim, C.K., and Lee, J.M., Inferring the genetic determinants of fruit colors in tomato by carotenoid profiling, Molecules, 2017, vol. 22, p. 764. https://doi.org/10.3390/molecules22050764
D'Andrea, L. and Rodriguez-Concepcion, M., Manipulation of plastidial protein quality control components as a new strategy to improve carotenoid contents in tomato fruit, Front. Plant Sci., 2019, vol. 10, p. 1071. https://doi.org/10.3389/fpls.2019.01071
Slugina, M.A., Efremov, G.I., Shchennikova, A.V., and Kochieva, E.Z., Characterization of RIN isoforms and their expression in tomato fruit ripening, Cells, 2021, vol. 10, p. 1739. https://doi.org/10.3390/cells10071739
Karlova, R., Chapman, N., David, K., Angenent, G.C., Seymour, G.B., and de Maagd, R.A., Transcriptional control of fleshy fruit development and ripening, J. Exp. Bot., 2014, vol. 65, p. 4527. https://doi.org/10.1093/jxb/eru316
Fujisawa, M., Shima, Y., Higuchi, N., Nakano, T., Koyama, Y., Kasumi, T., and Ito, Y., Direct targets of the tomato-ripening regulator RIN identified by transcriptome and chromatin immunoprecipitation analyses, Planta, 2012, vol. 235, p. 1107. https://doi.org/10.1007/s00425-011-1561-2
Fujisawa, M., Nakano, T., Shima, Y., and Ito, Y., A large-scale identification of direct targets of the tomato MADS box transcription factor RIPENING INHIBITOR reveals the regulation of fruit ripening, Plant Cell, 2013, vol. 25, p. 371. https://doi.org/10.1105/tpc.112.108118
Qin, G., Wang, Y., Cao, B., Wang, W., and Tian, S., Unraveling the regulatory network of the MADS box transcription factor RIN in fruit ripening, Plant J., 2012, vol. 70, p. 243. https://doi.org/10.1111/j.1365-313X.2011.04861.x
Fujisawa, M. and Ito, Y., The regulatory mechanism of fruit ripening revealed by analyses of direct targets of the tomato MADS-box transcription factor RIPENING INHIBITOR, Plant Signaling Behav., 2013, vol. 8, p. e24357. https://doi.org/10.4161/psb.24357
Fantini, E., Falcone, G., Frusciante, S., Giliberto, L., and Giuliano, G., Dissection of tomato lycopene biosynthesis through virus-induced gene silencing, Plant Physiol., 2013, vol. 163, p. 986. https://doi.org/10.1104/pp.113.224733
ACKNOWLEDGMENTS
This work was supported by the Russian Science Foundation, project no. 19-16-00016, and the Ministry of Science and Higher Education of the Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by N. Balakshina
The original online version of this article was revised: Due to a retrospective Open Access order.
Supplementary Information
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Efremov, G.I., Dzhos, E.A., Ashikhmin, A.A. et al. Effect of the Carotenoid Content and Activity of the Carotene cis-trans Isomerase CRTISO on Tomato Fruit Color. Russ J Plant Physiol 69, 64 (2022). https://doi.org/10.1134/S1021443722040045
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S1021443722040045