Abstract
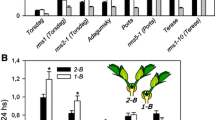
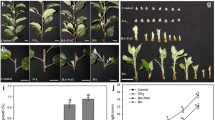
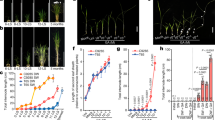
The interactions of branches in relation to the transports of IAA export activity (IEA) were studied in pea plants of the semidwarf cv. Adagumsky having a strong apical dominance (AD) and the dwarf cv. Porta with a weakened AD. In a model system of two-branched seedlings, the branches of cv. Adagumsky competitively suppressed the growth and IEA of each other, but that is not the case with cv. Porta. The root supply with GA3 inhibits the outgrowth of axillary buds at basal node 2 in cv. Porta seedlings, thus enhancing AD, and led to establishing a 1.5 fold different IEA in the shoots between two-branched and one-branched seedlings, making it similar to cv. Adagumsky. Thus, the emerging role of GAs as a systemic signal in regulation of AD and correlative inhibition is suggested. Molecular mechanisms of GA involvement in these processes are discussed.





Similar content being viewed by others
REFERENCES
Kotov, A.A., Kotova, L.M., and Romanov, G.A., Signaling network regulating plant branching: recent advances and new challenges, Plant Sci., 2021, vol. 307, art. ID 110880. https://doi.org/10.1016/j.plantsci.2021.110880
Wang, L. and Zhang, Q., Boosting rice yield by fine-tuning SPL gene expression, Trends Plant Sci., 2017, vol. 22, p. 643. https://doi.org/10.1016/j.tplants.2017.06.004
Adamowski, M. and Friml, J., PIN-dependent auxin transport: action, regulation, and evolution, Plant Cell, 2015, vol. 27, p. 20.
Tanaka, M., Takei, K., Kojima, M., Sakakibara, H., and Mori, H., Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance, Plant J., 2006, vol. 45, p. 1028.
Rameau, C., Bertheloot, J., Leduc, N., Andrieu, B., Sakr, S., and Foucher, F., Multiple pathways regulate shoot branching, Front. Plant Sci., 2015, vol. 5, p. 741. https://doi.org/10.3389/fpls.2014.00741
Ligerot, Y., de Saint Germain, A., Waldie, T., Troadec, C., Citerne, S., Kadakia, N., Pillot, J.P., Prigge, M., Aubert, G., Bendahmane, A., Leyser, O., Estelle, M., Debellé, F., and Rameau, C., The pea branching RMS2 gene encodes the PsAFB4/5 auxin receptor and is involved in an auxin-strigolactone regulation loop, PLoS Genet., 2017, vol. 13, p. e1007089. https://doi.org/10.1371/journal.pgen.1007089
Miyawaki, K., Matsumoto-Kitano, M., and Kakimoto, T., Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin, and nitrate, Plant J., 2004, vol. 37, p. 128.
Müller, D., Waldie, T., Miyawaki, K., To, J.P., Melnyk, C.W., Kieber, J.J., Kakimoto, T., and Leyser, O., Cytokinin is required for escape but not release from auxin mediated apical dominance, Plant J., 2015, vol. 82, p. 874.
Azarakhsh, M., Kirienko, A.N., Zhukov, V.A., Lebedeva, M.A., Dolgikh, E.A., and Lutova, L.A., KNOTTED1-LIKE HOMEOBOX 3: a new regulator of symbiotic nodule development, J. Exp. Bot., 2015, vol. 66, p. 7181. https://doi.org/10.1093/jxb/erv414
Zhang, K., Wang, R., Zi, H., Li, Y., Cao, X., Li, D., Guo, L., Tong, J., Pan, Y., and Jiao, Y., AUXIN RESPONSE FACTOR3 regulates floral meristem determinacy by repressing cytokinin biosynthesis and signaling, Plant Cell, 2018, vol. 30, p. 324. https://doi.org/10.1105/tpc.17.00705
Duan, J., Yu, H., Yuan, K., Liao, Z., Meng, X, Jing, Y., Liu, G., Chu, J., and Li, J., Strigolactone promotes cytokinin degradation through transcriptional activation of CYTOKININ OXIDASE/DEHYDROGENASE 9 in rice, Proc. Natl. Acad. Sci. U.S.A., 2019, vol. 116, p. 14319. https://doi.org/10.1073/pnas.1810980116
Ni, J., Zhao, M.L., Chen, M.S., Pan, B.Z., Tao, Y.B., and Xu, Z.F., Comparative transcriptome analysis of axillary buds in response to the shoot branching regulators gibberellin A3 and 6-benzyladenine in Jatropha curcas, Sci. Rep., 2017, vol. 7, p. 11417. https://doi.org/10.1038/s41598-017-11588-0
Roman, H., Girault, T., Barbier, F., Péron, T., Brouard, N., Pěnčík, A., Novák, O., Vian, A., Sakr, S., Lothier, J., Le Gourrierec, J., and Leduc, N., Cytokinins are initial targets of light in the control of bud outgrowth, Plant Physiol., 2016, vol. 172, p. 489. https://doi.org/10.1104/pp.16.00530
Barbier, F., Péron, T., Lecerf, M., Perez-Garcia, M.D., Barrière, Q., Rolčík, J., Boutet-Mercey, S., Citerne, S., Lemoine, R., Porcheron, B., Roman, H., Leduc, N., Le Gourrierec, J., Bertheloot, J., and Sakr, S., Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrid, J. Exp. Bot., 2015, vol. 66, p. 2569. https://doi.org/10.1093/jxb/erv047
Ljung, K., Nemhauser, J.L., and Perata, P., New mechanistic links between sugar and hormone signaling networks, Curr. Opin. Plant Biol., 2015, vol. 25, p. 130. https://doi.org/10.1016/j.pbi.2015.05.022
Di, D.W., Wu, L., Zhang, L., An, C.W., Zhang, T.Z., Luo, P., Gao, H.H., Kriechbaumer, V., and Guo, G.Q., Functional roles of Arabidopsis CKRC2/YUCCA8 gene and the involvement of PIF4 in the regulation of auxin biosynthesis by cytokinin, Sci. Rep., 2016, vol. 6, p. 36866. https://doi.org/10.1038/srep36866
Jones, B., Gunneras, S.A., Petersson, S.V., Tarkowski, P., Graham, N., May, S., Dolezal, K., Sandberg, G., and Ljung, K., Cytokinin regulation of auxin synthesis in Arabidopsis involves a homeostatic feedback loop regulated via auxin and cytokinin signal transduction, Plant Cell, 2010, vol. 22, p. 2956.
Kushwah, S. and Laxmi, A., The interaction between glucose and cytokinin signal transduction pathway in Arabidopsis thaliana, Plant, Cell Environ., 2014, vol. 37, p. 235. https://doi.org/10.1111/pce.12149
Brenner, W.G. and Schmülling, T., Transcript profiling of cytokinin action in Arabidopsis roots and shoots discovers largely similar but also organspecific responses, BMC Plant Biol., 2012, vol. 12, p. 112.
Willige, B.C., Isono, E., Richter, R., Zourelidou, M., and Schwechheimer, C., Gibberellin regulates PIN-FORMED abundance and is required for auxin transport-dependent growth and development in Arabidopsis thaliana, Plant Cell, 2011, vol. 23, p. 2184. https://doi.org/10.1105/tpc.111.086355
Bai, F. and DeMason, D.A., Hormone interactions and regulation of Unifoliata, PsPK2, PsPIN1, and LE gene expression in pea (Pisum sativum) shoot tips, Plant Cell Physiol., 2006, vol. 47, p. 935.
Huang, F., Zago, M.K., Abas, L., van Marion, A., Galván-Ampudia, C.S., and Offringa, R., Phosphorylation of conserved PIN motifs directs Arabidopsis PIN1 polarity and auxin transport, Plant Cell, 2010, vol. 22, p. 1129.
Salanenka, Y., Verstraeten, I., Löfke, C., Tabata, K., Naramoto, S., Glanc, M., and Friml, J., Gibberellin DELLA signaling targets the retromer complex to redirect protein trafficking to the plasma membrane, Proc. Natl. Acad. Sci. U.S.A., 2018, vol. 115, p. 3716.
Yoshida, H., Ueguchi-Tanaka, M., and Matsuoka, M., Regulatory networks acted upon by the GID-DELLA system after perceiving gibberellins, in The Enzymes, Vol. 35: Signaling Pathways in Plants, Mashida, Y., et al., Eds., Amsterdam: Elsevier, 2014, vol. 35, ch. 1, p. 1. https://doi.org/10.1016/B978-0-12-801922-1.00001-4
Kotov, A.A. and Kotova, L.M., Auxin–cytokinin interactions in the regulation of correlative inhibition in two-branched pea seedlings, J. Exp. Bot., 2018, vol. 69, p. 2967. https://doi.org/10.1093/jxb/ery117
Kotov, A.A. and Kotova, L.M., Correlative inhibition between branches in two-branched pea seedlings is cultivar-dependent, J. Plant Growth Regul., 2019, vol. 38, p. 132. https://doi.org/10.1007/s00344-018-9821-z
Morris, S.E., Turnbull, C.G.N., Murfet, I.C., and Beveridge, C.A., Mutational analysis of branching in pea. Evidence that Rms1 and Rms5 regulate the same novel signal, Plant Physiol., 2001, vol. 126, p. 1205.
Bennett, T., Hines, G., van Rongen, M., Waldie, T., Sawchuk, M.G., Scarpella, E., Ljung, K., and Leyser, O., Connective auxin transport in the shoot facilitates communication between shoot apices, PLoS Biol., 2016, vol. 14, p. e1002446. https://doi.org/10.1371/journal.pbio.1002446
Dun, E.A., de Saint Germain, A., Rameau, C., and Beveridge, C.A., Antagonistic action of strigolactone and cytokinin in bud outgrowth control, Plant Physiol., 2012, vol. 158, p. 487.
Leivar, P. and Monte, E., PIFs: systems integrators in plant development, Plant Cell, 2014, vol. 26, p. 56. https://doi.org/10.1105/tpc.113.120857
Shi, H., Liu, W., Wei, Y., and Ye, T., Integration of auxin/indole-3-acetic acid 17 and RGA-LIKE3 confers salt stress resistance through stabilization by nitric oxide in Arabidopsis, J. Exp. Bot., 2017, vol. 68, p. 1239. https://doi.org/10.1093/jxb/erw508
Kolachevskaya, O.O., Lomin, S.N., Arkhipov, D.V., and Romanov, G.A., Auxins in potato: molecular aspects and emerging roles in tuber formation and stress resistance, Plant Cell Rep., 2019, vol. 38, p. 681.
Moubayidin, L., Perilli, S., Dello Ioio, R., Di Mambro, R., Costantino, P., and Sabatini, S., The rate of cell differentiation controls the Arabidopsis root meristem growth phase, Curr. Biol., 2010, vol. 20, p. 1138.
Iqbal, N., Nazar, R., Khan, M.I.R., Masood, A., and Khan, N.A., Role of gibberellins in regulation of source-sink relations under optimal and limiting environmental conditions, Curr. Sci., 2011, vol. 100, p. 998.
Rinne, P.L., Paul, L.K., Vahala, J., Kangasjärvi, J., and van der Schoot, C., Axillary buds are dwarfed shoots that tightly regulate GA pathway and GA-inducible 1,3-β-glucanase genes during branching in hybrid aspen, J. Exp. Bot., 2016, vol. 67, p. 5975.
Rabot, A., Portemer, V., Péron, T., Mortreau, E., Leduc, N., Hamama, L., Coutos-Thévenot, P., Atanassova, R.S., and Le Gourrierec, J., Interplay of sugar, light and gibberellins in expression of Rosa hybrida vacuolar invertase 1 regulation, Plant Cell Physiol., 2014, vol. 55, p. 1734. https://doi.org/10.1093/pcp/pcu106
Salam, B.B., Barbier, F., Danieli, R., Teper-Bamnolker, P., Ziv, C., Spıíchal, L., Aruchamy, K., Shnaider, Y., Leibman, D., Shaya, F., Weissberg, M.C., Gal-On, A., Jiang, J., Ori, N., Beveridge, C., and Eshel, D., Sucrose promotes stem branching through cytokinin, Plant Physiol., 2021, p. 1. https://doi.org/10.1093/plphys/kiab003
Kotov, A.A. and Kotova, L.M., The contents of auxins and cytokinins in pea internodes as related to the growth of lateral buds, J. Plant Physiol., 2000, vol. 156, p. 438.
Roitsch, T. and Ehneb, R., Regulation of source/sink relations by cytokinins, Plant Growth Regul., 2000, vol. 32, p. 359.
Biemelt, S., Tschiersch, H., and Sonnewald, U., Impact of altered gibberellin metabolism on biomass accumulation, lignin biosynthesis, and photosynthesis in transgenic tobacco plants, Plant Physiol., 2004, vol. 135, p. 254. https://doi.org/10.1104/pp.103.036988
ACKNOWLEDGMENTS
We thank Dr. Catherine Rameau (INRA Centre de Versailles-Grignon, France) very much for providing the seeds of pea wild-type cv. Porta.
Funding
The research was carried out within the state assignment of Ministry of Science and Higher Education of the Russian Federation (no. 121033000137-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of humans or animals. This article does not contain any studies involving humans or animals performed by any of the authors.
Additional information
Abbreviations: 2-B and 1-B—two- and one-branched plants, respectively; AD—apical dominance; ARF—AUXIN RESPONSE FACTOR; Aux/IAA—AUXIN/INDOLE-3-ACETIC ACID INDUCIBLE protein, a transcriptional repressor of auxin-inducible genes; CCD7/MAX3/RMS5 and CCD8/MAX4/RMS1—carotenoid cleavage dioxygenases of Arabidopsis/pea; CI—correlative inhibition; CK—cytokinin; CKX—cytokinin oxidase; D14/AtD14/RMS3—rice/Arabidopsis/pea strigolactone receptor; D53/SMXL—SUPPRESSOR OF MAX2 1-LIKE protein, first target protein in strigolactone signaling; DELLA—a conserved DELLA domain in GA signaling proteins; IEA—IAA export activity; IPT—adenosine phosphate-ISOPENTENYLTRANSFERASE; LOG—Lonely Guy, enzyme which convert cytokinin nucleotides in free basis; MAX2/RMS4/D3—the Arabidopsis/pea/rice F-box protein, a component of SCF E3 ubiquitin ligase complex; PID—Ser/Thr kinase PINOID; PIF—PHYTOCHROME-INTERACTING FACTOR protein; PIN—membrane auxin efflux carriers of the family PINFORMED; PM—plasma membrane; RGA— REPRESSOR OF GA, DELLA protein; SEE—standard error of the estimate; SL—strigolactone; Sug—sugars; TAA/TAR—TRYPTOPHAN AMINOTRANSFERASE related protein; TIR1/AFB—TRANSPORT INHIBITOR RESPONSE 1/AUXIN SIGNALING F‑BOX PROTEIN, auxin receptor; type B RR—a positive type B RESPONSE REGULATOR of cytokinin response; X-CK—xylem cytokinin; YUC—YUCCA, flavin monooxygenase-like enzyme.
Rights and permissions
About this article
Cite this article
Kotov, A.A., Kotova, L.M. In What Way Do Gibberellins Increase Apical Dominance and Correlative Inhibition?. Russ J Plant Physiol 69, 22 (2022). https://doi.org/10.1134/S102144372202008X
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S102144372202008X