Abstract
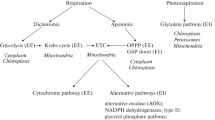
Plants of spring wheat (Triticum aestivum L.) and winter rye (Secale cereale L.) pursuing different phenological strategies were studied. Respiratory activity, ratio of respiratory pathways, and effect of the alterative pathway (AP) on the YATP/glucose coefficient, which expresses the energy efficiency of respiration (EER), were studied over the leaf ontogeny. At 20°C, the respiratory capacity of the wheat leaf was higher than that of rye due to the decrease in rye metabolism in an autumn period of vegetation. Respiration decreased with age and relative growth rate (RGR) of the leaf. In the young leaf whose area was 20–30% of the final value, respiration mainly proceeded by the cytochrome pathway because of energy expenses for de novo synthesis. In the spring wheat leaf, the AP fraction of its respiration increased from 25 to 40% with age; this indicates the AP belonging to maintenance respiration component. In the mature rye leaf, the AP contribution decreased from 35 to 15% of a total respiration that maintained EER during plant adaptation to low temperatures. A change in the direction of respiratory gradient along the leaf was also found. In leaves of different age, the meristematically active zone manifested the greatest values of such indices as rate of respiration, fraction of AP (up to 45% of a total respiration), and rate of thermogenesis; this shows participation of alternative respiration in energy dissipation and energy balance control. Altogether, the value YATP/glucose did not change at the level of wheat and rye leaf of different ages. On average, it was 20 mole ATP/mole glucose, which is one third lower than the theoretically assumed value. This may be interpreted so that the metabolic level corresponds to environmental conditions and is adapted to them.






Similar content being viewed by others
REFERENCES
Mokronosov, A.T., Mesostructure and functional activity of the photosynthetic apparatus, in Mezostruktura i funktsional’naya aktivnost' fotosinteticheskogo apparata (Mesostructure and Functional Activity of the Photosynthetic Apparatus), Mokronosov, A.T., Borzenkova, R.A., Tsel’niker, Yu.L., and Nekrasova, G.F., Eds., Sverdlovsk, 1978, pp. 5–31.
Mokoronosov, A.T., Ontogeneticheskii aspekt fotosinteza (Ontogenetic Aspect of Photosynthesis), Moscow: Nauka, 1981.
Mokronosov, A.T., Fotosinteticheskaya funktsiya i tselostnost' rastitel’nogo organizma, 42-e Timiryazevskoe chteniye (Photosynthetic Function and Integrity of the Plant Organism, the 42nd Timiryazev Lecture), Moscow: Nauka, 1983.
Semikhatova, O.A., Energy aspects of the integration of physiological processes in plants, Sov. Plant Physiol., 1980, vol. 27, pp. 1005–1017.
Garmash, E.V., Mitochondrial respiration of the photosynthesizing cell, Russ. J. Plant Physiol., 2016, vol. 63, pp. 13–25.
Golovko, T.K., Dykhanie rastenii (fiziologicheskie aspekty) (Plant Respiration (Physiological Aspects)), St. Petersburg: Nauka, 1999.
Li, L., Nelson, C.J., Trösch, J., Castleden, I., Huang, S., and Millar, A.H., Protein degradation rate in Arabidopsis thaliana leaf growth and development, Plant Cell, 2017, vol. 29, pp. 207–228.
Millenaar, F.F. and Lambers, H., The alternative oxidase: in vivo regulation and function, Plant Biol., 2003, vol. 2, pp. 2–15.
Semikhatova, O.A., Energetika dykhaniya v norme i pri ekologicheskom stresse, 48-e Timiryazevskoe chteniye (Respiratory Energy in Normal and under Environmental Stress, the 48th Timiryazev Lecture), Moscow: Nauka, 1990.
Rakhmankulova, Z.F., Levels of energy metabolism control in plants, Vestn. Bashkir. Gos. Univ., 2009, vol. 14, no. 3 (I), pp. 1141–1154.
Noguchi, K., Effects of light intensity and carbohydrate status on leaf and root respiration, in Plant Respiration: From Cell to Ecosystem, Ch. 5, Lambers, H. and Ribas-Carbo, M., Eds., Dordrecht: Springer, 2005, pp. 63–83.
Florez-Sarasa, I.D., Bouma, T.J., Medrano, H., Azcon-Bieto, J., and Ribas-Carbo, M., Contribution of the cytochrome and alternative pathways to growth respiration and maintenance respiration in Arabidopsis thaliana, Physiol. Plant., 2007, vol. 129, pp. 143–151.
Priault, P., Vidal, G., de Paepe, R., and Ribas-Carbo, M., Leaf age-related changes in respiratory pathways are dependent on complex I activity in Nicotiana sylvestris, Physiol. Plant., 2007, vol. 129, pp. 152–162.
Ivanova, T.I., Kirpichnikova, O.V., Sherstneva, O.A., and Yudina, O.S., Annual cycle of respiration in the leaves of evergreen plants, Russ. J. Plant Physiol., 1998, vol. 45, pp. 786–793.
Golovko, T.K. and Pystina, N.V., The alternative respiration pathway in leaves of Rhodiola rosea and Ajuga reptans: presumable physiological role, Russ. J. Plant Physiol., 2001, vol. 48, pp. 733–740.
Shugaev, A.G., Vyskrebentseva, A.I., and Shugaeva, N.A., Seasonal changes in the activity of mitochondrial oxidases detected by the traditional inhibitor analysis in disks cut from mature sugar beet leaves, Russ. J. Plant Physiol., 1998, vol. 45, pp. 574–581.
Tarchevskii, I.A., Metabolizm rastenii pri stresse (izbrannye trudy) (Plant Metabolism under Stress (Selected Works)), Kazan: Fen, 2001.
Kikuzawa, K., Leaf phenology as an optimal strategy for carbon gain in plants, Can. J. Bot., 1995, vol. 73, pp. 158–163.
Radford, P.J., Growth analysis formulae—their use and abuse, Crop Sci., 1967, vol. 7, pp. 171–175.
Møller, I.M., Berczi, A., Plas van der L.H.W., and Lambers, H., Measurement of the activity and capacity of the alternative pathway in intact plant tissue: identification of problems and possible solution, Physiol. Plant., 1988, vol. 72, pp. 642–649.
Amthor, J.S., The McCree-de Wit-Penning de Vries-Thornley respiration paradigms: 30 years later, Ann. Bot., 2000, vol. 86, pp. 1–20.
Bradford, M.M., A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding, Anal. Biochem., 1976, vol. 72, pp. 248–254.
Moskalev, A.A. and Novakovskii, A.B., Statisticheskie metody v ekologii s ispol’zovaniem R, Statistica, Excel i SPSS (Statistical Methods in Ecology Using R, Statistica, Excel, and SPSS), Syktyvkar: Syktyvkar Gos. Univ., 2014.
Edwards, J.M., Roberts, T.H., and Atwell, B.J., Quantifying ATP turnover in anoxic coleoptiles of rice (Oryza sativa) demonstrates preferential allocation of energy to protein synthesis, J. Exp. Bot., 2012, vol. 63, pp. 4389–4402.
Grabel'nykh, O.I., Mitochondrial energy dispersive systems of plants under the action of low temperatures, Extended Abstract of Doctoral (Biol.) Dissertation, Irkutsk: Sib. Inst. Plant Physiol. Biochem., Sib. Branch, Russ. Acad. Sci., 2014.
Garmash, E.V., Malyshev, R.V., Shelyakin, M.A., and Golovko, T.K., Activities of respiratory pathways and the pool of nonstructural carbohydrates in greening leaves of spring wheat seedlings, Russ. J. Plant Physiol., 2014, vol. 56, pp. 160–168.
Garmash, E.V. and Golovko, T.K., Effect of growth rate of barley plants grown at different temperatures and mineral nutrition levels on alternative respiratory pathway activity, Fiziol. Biokhim. Kult. Rast., 2011, vol. 43, pp. 113–121.
Shugaeva, N.A., Vyskrebentseva, E.I., Orekhova, S.O., and Shugaev, A.G., Effect of water deficit on respiration of conducting bundles in leaf petioles of sugar beet, Russ. J. Plant Physiol., 2007, vol. 54, pp. 329–335.
Sayed, M.A., Umekawa, Y., and Kikukatsu, I., Metabolic interplay between cytosolic phosphoenolpyruvate carboxylase and mitochondrial alternative oxidase in thermogenic skunk cabbage, Symplocarpus renifolius, Plant Signal. Behav., 2016, vol. 11, no. 11: e1247138. https://doi.org/10.1080/15592324.2016.1247138
Shane, M.W., Cramer, M.D., Funayama-Noguchi, S., Cawthray, G.R., Millar, A.H., Day, D.A., and Lambers, H., Developmental physiology of cluster-root carboxylate synthesis and exudation in Harsh Hakea. Expression of phosphoenolpyruvate carboxylase and the alternative oxidase, Plant Physiol., 2004, vol. 135, pp. 549–560.
Funding
The work was carried out in terms of the budget theme “Physiology and Stress-Resistance of Plant Photosynthesis and that of Poikilohydric Photoautotrophs under Conditions of the North,” project no. GR AAAA-A17-117033010038-7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by A. Aver’yanov
Abbreviations:
AOX—alternative oxidase; CP, AP—cytochrome and alternative respiration pathway, respectively; EER—energy efficiency of respiration; RGR—leaf relative growth rate; SHAM—salicylhydroxamic acid; Vcyt, Valt, Vres—rates of CP, AP, and residual respiration, respectively; Vt—dark respiration rate measured as the O2 uptake rate; YATP/glucose—coefficient of glucose oxidation efficiency for ATP formation.
Rights and permissions
About this article
Cite this article
Garmash, E.V. Respiration and Involvement of an Alternative Pathway as Related to Age and Phenological Strategy of the Leaf. Russ J Plant Physiol 66, 403–413 (2019). https://doi.org/10.1134/S102144371903004X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S102144371903004X