Abstract
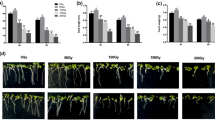
This study examines the effects of ionizing radiation (control, 4 × 1016, 6 × 1016, 9.5 × 1016, and 15 × 1016 Ar+/cm2) on some physio-biochemical and molecular responses of highland barley (Hordeum vulgare L. ssp. vulgare) under salt stress for 0, 24, 48, and 96 h. The growth parameters of highland barley were the highest at the dose of 9.5 × 1016 Ar+/cm2, but the lowest at 15 × 1016 Ar+/cm2 dose. The malondialdehyde (MDA) content increased with increasing irradiation dose and peaked at 15 × 1016 Ar+/cm2 during stress treatments. The activities of antioxidant enzymes and proline accumulation showed different changes than MDA following ion beam irradiation toward stress conditions, at the dose of 15 × 1016 Ar+/cm2, antioxidant enzyme activities and proline content were the lowest compared with their corresponding controls, while at the dose of 9.5 × 1016 Ar+/cm2 antioxidant enzyme activities and proline content were the highest. Moreover, the expression of salt-related gene followed the same pattern as that of the antioxidant enzymes. Our results suggest that the dose of 9.5 × 1016 Ar+/cm2 alleviates salt stress by modulating the physio-biochemical responses and eliciting the stress signal transduction in plants.
Similar content being viewed by others
Abbreviations
- CAT:
-
catalase
- MDA:
-
malondialdehyde
- POD:
-
peroxidase
- SOD:
-
superoxide dismutase
References
Bose, J., Xie, Y., and Shen, W., Haem oxygenase modifies salinity tolerance in Arabidopsis by controlling K+ retention via regulation of the plasma membrane H+- ATPase and by altering SOS1 transcript levels in roots, J. Exp. Bot., 2013, vol. 64, pp. 471–481.
Qiu, J., The third pole, Nature, 2008, vol. 454, pp. 393–396.
Gama, P.B.S., Inanaga, S., Tanaka, K., and Nakazawa, R., Physiological response of common bean (Phaseolus vulgaris L.) seedlings to salinity stress, Afr. J. Biotechnol., 2007, vol. 6, pp. 79–88.
Wang, J., Li, H.R., Li, Y.H., Yu, J.P., Yang, L.S., Feng, F.J., and Chen, Z., Speciation, distribution, and bioavailability of soil selenium in the Tibetan Plateau Kashin-Beck disease area-a case study in Songpan County, Sichuan Province, China, Biol. Trace Elem. Res., 2013, vol. 156, pp. 367–375.
Li, W.H., Xiao, X.L., Zhang, W.H., Zheng, J.M., and Luo, Q.G., Compositional, morphological, structural and physicochemical properties of starches from seven naked barley cultivars grown in China, Food Res. Int., 2014, vol. 58, pp. 7–14.
Schulte, D., Timothy, J.C., Graner, A., Langridge, P., Matsumoto, T., Muehlbauer, G., Sato, K., Schulman, A.H., Waugh, R., Wise, R.P., and Stein, N., The International Barley Sequencing Consortium—at the threshold of efficient access to the barley genome, Plant Physiol., 2009, vol. 149, pp. 142–147.
Barkla, B.J., Castellanos-Cervantes, T., Diaz de León, J.L., Matros, A., Mock, H.P., Perez-Alfocea, F., Salekdeh, G.H., Withzel, K., and Zorb, C., Elucidation of salt stress defense and tolerance mechanisms of crop plants using proteomics—current achievements and perspectives, Proteomics, 2013, vol. 13, pp. 1885–1900.
Yang, Q., Chen, Z.Z., Zhou, X.F., Yin, H.B., Li, X., Xin, X.F., Hong, X.H., Zhu, J.K., and Gong, Z.Z., Overexpression of SOS (Salt Overly Sensitive) genes increases salt tolerance in transgenic Arabidopsis, Mol. Plant, 2009, vol. 2, pp. 22–31.
Shi, H., Quintero, F.J., Pardo, J.M., and Zhu, J.K., The putative plasma membrane Na +/H + antiporter SOS1 controls long distance Na+ transport in plants, Plant Cell, 2002, vol. 14, pp. 465–477.
Liu, J., Ishitani, M., Halfter, U., Kim, C.S., and Zhu, J.K., The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance, Proc. Natl. Acad. Sci. USA, 2000, vol. 97, pp. 3730–3734.
Liu, J. and Zhu, J.K., A calcium sensor homolog required for plant salt tolerance, Science, 1998, vol. 280, pp. 1943–1945.
Arase, S., Hase, Y., Abe, J., Kasai, M., Yamada, T., Kitamura, K., Narumi, I., Tanaka, A., and Kanazawa, A., Optimization of ion-beam irradiation for mutagenesis in soybean: effects on plant growth and production of visibly altered mutants, Plant Biotechnol., 2011, vol. 28, pp. 323–329.
Jain, S.M., Mutagenesis in crop improvement under the climate change, Rom. Biotech. Lett., 2010, vol. 2, suppl., pp. 88–106.
Giannopolitis, C.N. and Ries, S.K., Superoxide dismutases. I. Occurrence in higher plants, Plant Physiol., 1977, vol. 59, pp. 309–314.
Bergmeyer, N., Methode Enzymaticchen Analyse, Berlin: Akademie-Verlag, 1970.
Zhang, J. and Kirkham, M.B., Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species, Plant Cell Physiol., 1994, vol. 35, pp. 785–791.
Bates, L.S., Waldren, R.P., and Teare, I.D., Rapid determination of free proline for water-stress studies, Plant Soil, 1973, vol. 39, pp. 205–207.
Qi, W.C., Zhang, L., Xu, H.B., Wang, L., and Jiao, Z., Physiological and molecular characterization of the enhanced salt tolerance induced by low-dose gamma irradiation in Arabidopsis seedlings, Biochem. Biophys. Res. Commun., 2014, vol. 450, pp. 1010–1015.
Shalata, A. and Neumann, P.M., Exogenous ascorbic acid (vitamin C) increases resistance to salt stress and reduces lipid peroxidation, J. Exp. Bot., 2001, vol. 52, pp. 2207–2211.
Zhang, N. and Yu, L., Effect of N+ ion implantation on antioxidase activity in Blakeslea trispora, Radiat. Phys. Chem., 2008, vol. 77, pp. 1046–1049.
Liu, Z.Q., Gu, W.B., and Li, W.J., Effect of heavy-ion beams irradiation on survival rate and antioxidant enzymes of sweet sorghum seedlings, Agric. Sci. Technol., 2012, vol. 11, pp. 2257–2268.
Olivieri, G., Bodycote, J., and Wolff, S., Adaptive response of human lymphocytes to low concentrations of radioactive thymidine, Science, 1984, vol. 4636, pp. 594–597.
Yang, G., Mei, T., Yuan, H., Zhang, W., Chen, L., Xue, J., Wu, L., and Wang, Y., Bystander/abscopal effects induced in intact Arabidopsis seeds by lowenergy heavy-ion radiation, J. Radiat. Res., 2008, vol. 170, pp. 372–380.
Chen, H., Li, F., Yuan, H., Xiao, X., Yang, G., and Wu, L., Abscopal signals mediated bio-effects in lowenergy ion irradiated Medicago truncatula seeds, J. Radiat. Res., 2010, vol. 51, pp. 651–656.
Zhu, J.K., Regulation of ion homeostasis under salt stress, Curr. Opin. Plant Biol., 2003, vol. 6, pp. 441–445.
Semsang, N. and Deng, L., Induction of antioxidant enzyme activity and lipid peroxidation level in ionbeam- bombarded rice seeds, Nucl. Instrum. Methods Phys. Res., Sect. B, 2013, vol. 307, pp. 603–609.
Ashraf, M. and Foolad, M.R., Roles of glycine betaine and proline in improving plant abiotic stress resistance, Ecotox. Environ. Safe., 2007, vol. 59, pp. 206–216.
Nagata, T., Yamada, H., and Du, Z., Microarray analysis of genes that respond to gamma-irradiation in Arabidopsis, J. Agric. Food Chem., 2005, vol. 53, pp. 1022–1030.
Kim, D.S., Kim, J.B., and Goh, E.J., Antioxidant response of Arabidopsis plants to gamma irradiation: genome-wide expression profiling of the ROS scavenging and signal transduction pathways, J. Plant Physiol., 2011, vol. 168, pp. 1960–1971.
Shereen, A., Ansari, R., and Mumtaz, S., Impact of gamma irradiation induced changes on growth and physiological responses of rice under saline conditions, Pak. J. Bot., 2009, vol. 41, pp. 2487–2495.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Wang, X.J., Jiao, Z., Liang, J.Q. et al. Ar+ beam implantation causes enhancement of salt stress tolerance in highland barley. Russ J Plant Physiol 64, 749–757 (2017). https://doi.org/10.1134/S1021443717050144
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443717050144