Abstract
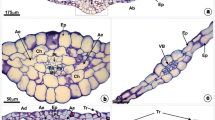
Anatomic–morphological and functional features of sea aster (Aster tripolium L.) leaves were investigated in the field study on the northern limit of A. tripolium’s range of distribution, using plants growing in the intertidal zone on the Pomorsky coast of the White Sea. The largest leaf area was found in plants grown close to the shore, near the high tide mark. In plants grown near the low tide mark, the leaf area decreased fourfold, while the leaf thickness increased by 30%. The plants sampled near the low and high tide marks showed different rates of photosynthesis and transpiration; they also differed in stomatal conductance and the number of stomata on the upper and lower leaf sides. Analysis of carbon dioxide plots of photosynthesis revealed the habitat-related differences in photosynthetic characteristics. Under saturating concentrations of carbon dioxide, the plants grown near the shore exhibited larger rates of photosynthetic electron transport and carboxylation. Characteristics of plants in the examined transect were found related to the local environmental conditions. A hypothesis is put forward that A. tripolium possesses an inducible CO2 concentrating mechanism during the growing season under northern summer conditions.
Similar content being viewed by others
Abbreviations
- CCM:
-
CO2-concentrating mechanism
- Ci :
-
inorganic carbon forms (CO2 + HCO3)
- CVC:
-
cell volume per chloroplast
- J max/V cmax :
-
ratio of maximal rates of electron transport and carboxylation
- P n :
-
net photosynthesis
References
Uedo, A., Kanechio, M., Uno, Y., and Inagaki, N., Photosynthetic limitations of a halophyte sea aster (Aster tripolium L.) under water stress and NaCl stress, J. Plant Res., 2003, vol. 116, pp. 65–70.
Ramani, B., Reeck, Th., Debez, A., Stelzer, R., Huchzermeyer, B., Schmidt, A., and Papenbrock, J., Aster tripolium and Sesuvium portulacastrum L.: two halophytes, two strategies to survive in saline habitats, Plant Physiol. Biochem., 2006, vol. 44, pp. 395–408.
Flowers, T., Galal, H., and Bromham, L., Evolution of halophytes: multiple origins of salt tolerance in land plants, Funct. Plant Biol., 2010, vol. 37, pp. 604–612.
Anderson, U., Samson, I., and Levinsh, G., Protection of photosynthesis in coastal salt marsh plants Aster tripolium and Hydrocotyle vulgaris in conditions of increased soil salinity, Environ. Exp. Biol., 2012, vol. 10, pp. 89–97.
Levinsh, G., Biological basis of biological diversity: physiological adaptation of plants to heterogeneous habitats along a sea coast, Acta Univ. Latv., Ser. Biol., 2006, vol. 710, pp. 53–79.
Shennan, C., Gupta, N.K., Gupta, S., and Hasegawa, H., Effect of NaCl salinity on photosynthetic rate, transpiration rate, and oxidative stress tolerance in contrasting wheat genotypes, Photosynthetica, 2005, vol. 43, pp. 609–613.
Huiskes, A.H.L., Koutstaal, B.P., Wielemaker-Van den Dool, A., and Markusse, M.M., A study on polymorphism in Aster tripolium L. (Sea Aster), Plant Biol., 2000, vol. 2, pp. 547–557.
Markovskaya, E.F., Sergienko, L.A., Shklyarevich, G.A., Sonina, A.V., Starodubtseva, A.A., and Smol’kova, O.V., Prirodnyi kompleks poberezh’ya Belogo morya (Natural Complex of the White Sea Coast), Petrozavodsk: Karel. Nauch. Tsentr, Ross. Akad. Nauk, 2010.
Uno, Y., Kanechi, M., Inagaki, N., Sugimoto, M., and Maekawa, S., The evaluation of salt tolerance during germination and vegetative growth of asparagus, table beet and sea aster, J. Jpn. Soc. Hortic. Sci., 1996, vol. 65, pp. 579–585.
Ramenskaya, M.L., Analiz flory Murmanskoi oblasti i Karelii (Analysis of Flora in Murmansk Oblast and Karelia), Leningrad: Nauka, 1983.
Zaslavskaya, N.V., Flora of the saline ecotopes of the White Sea West Coast, Extended Abstract of Cand. Sci. (Biol.) Dissertation, Petrozavodsk: Petrozavodsk. Gos. Univ., 2007.
Kravchenko, A.V., Konspekt flory Karelii (Flora of Karelia), Petrozavodsk: Karel. Nauch. Tsentr, Ross. Akad. Nauk, 2007.
Farquhar, G.D., Caemmerer, S., and Berry, J.A., A biochemical model of photosynthetic CO2 assimilation in leaves of C3 plants, Planta, 1980, vol. 149, pp. 78–90.
Caemmerer, S. and Farquhar, G.D., Some relationships between the biochemistry of photosynthesis and the gas exchange rates of leaves, Planta, 1981, vol. 153, pp. 376–387.
Harley, P.C. and Sharkey, T.D., An improved model of C3 photosynthesis at high CO2: reversed O2 sensitivity explained by lack of glycerate re-entry into the chloroplast, Photosynth. Res., 1991, vol. 27, pp. 169–178.
Parsons, R. and Ogston, S., Photosynthesis Assistant: Tools for Analysis of Photosynthetic Data, Versio. 1.1.2, Dundee, UK: Dundee Sci., 1999.
Furst, G.G., Metody anatomo-gistokhimicheskogo issledovaniya rastitel’nykh tkanei (Methods for Anatomical and Histochemical Investigations of Plant Tissues), Moscow: Nauka, 1979.
Mokronosov, A.T. and Borzenkova, R.A., Methods for quantifying the functional activity of photosynthetic tissues and organs, Tr. Prikl. Bot. Genet. Sel., 1978, vol. 61, no. 3, pp. 119–133.
Morozova, K.V., Gulyaeva, E.N., and Markovskaya, E.F., Anatomical and morphological characteristics of Aster tripolium L. on the White Sea Coast, Uch. Zapiski Petrozavodsk. Gos. Univ., Ser.: Estestv. Techn. Nauki, 2014, vol. 2, no. 8 (145), pp. 21–25.
Grigore, M.-N., Toma, C., and Bo caiu, M., Ecological implications of bulliform cells on halophytes, in salt and water stress natural conditions, Biol. Veg., 2010, vol. 56, no. 2, pp. 5–15.
Burkovskaya, E.V., Mesostructure of vascular plant leaves on the supralittoral of Japan Sea, Vestn. Krasnoyarsk. Gos. Agrar. Univ., 2008, no. 2, pp. 107–111.
Goryshina, T.K., Fotosinteticheskii apparat rastenii i usloviya sredy (Photosynthetic Apparatus of Plants and Environment), Leningrad: Leningr. Gos. Univ., 1989.
Voronkova, N.M., Burkovskaya, E.V., Bezdeleva, T.A., and Burundukova, O.L., Morphological and biological features of plants related to their adaptation to coastal habitats, Russ. J. Ecol., 2008, vol. 39, pp. 1–7.
Pronina, N.A., The organization and physiological role of the CO2–CM in microalgal photosynthesis, Russ. J. Plant Physiol., 2000, vol. 47, pp. 706–714.
Rascio, N., The underwater life of secondarily aquatic plants: some problems and solutions, Crit. Rev. Plant Sci., 2002, vol. 21, pp. 401–427. do. 10.1080/073526029104429.
Pederson, O., Colmer, T.D., and Sand-Jensen, K., Underwater photosynthesis of submerged plants–recent advances and methods, Front. Plant Sci., 2013, vol. 4, p. 140. do. 10.3389/fpls.2013.0014.
Hikosaka, K., Ishikawa, K., Borjigidai, A., Muller, O., and Onoda, Y., Temperature acclimation of photosynthesis: mechanisms involved in the changes in temperature dependence of photosynthetic rate Hill, J. Exp. Bot., 2006, vol. 57, pp. 291–302.
Markovskaya, E., Sonina, A., Sergienko, L., Morozova, K., and Elkina, N., Morphological and functional peculiarities of saltmarsh plants and epilithic lichens in tidal conditions of Russian Arctic seas, Czech. Polar Rep., 2014, vol. 4, pp. 168–177.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.F. Markovskaya, A.A. Kosobryukhov, K.V. Morozova, E.N. Gulyaeva, 2015, published in Fiziologiya Rastenii, 2015, Vol. 62, No. 6, pp. 847–853.
Rights and permissions
About this article
Cite this article
Markovskaya, E.F., Kosobryukhov, A.A., Morozova, K.V. et al. Photosynthesis and anatomic–morphological characteristics of sea aster leaves on the white sea coast. Russ J Plant Physiol 62, 830–836 (2015). https://doi.org/10.1134/S1021443715060126
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443715060126