Abstract
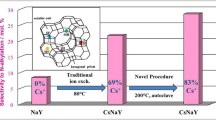
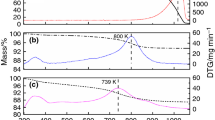
This study investigates the effects of the degree of ion exchange of sodium cations for cesium cations in FAU(Y) on its physicochemical properties. Using aqueous and solid-state ion exchange, a number of NaY samples with an exchange degree of sodium cations for cesium cations varying from 29 to 89% were prepared. The samples were examined by SEM, X-ray fluorescence, low-temperature nitrogen adsorption, XRD, NH3-TPD, IR spectroscopy of adsorbed chloroform, and 27Al MAS NMR. It was demonstrated that samples with exchange degrees up to 87% can be synthesized with their crystalline structure intact. The test of the catalytic properties of the synthesized samples in aniline alkylation with methanol showed a growth in the selectivity for N-alkylated products as the number and strength of basic sites were progressively increased. Impregnating the Cs-containing zeolites with CsOH was found to significantly enhance the operating stability of the samples and the yield of N-alkylated products, compared to CsNaY. The CsOH-modified catalysts with a Na+/Cs+ exchange degree of 54–77% proved to be the most active and stable in aniline alkylation with methanol: they provided aniline conversion of 81–88% and selectivity for N-alkylation products as high as 99.6–99.7 mol %.








Similar content being viewed by others
REFERENCES
Bordawekar, S.V. and Davis, R.J., J. Catal., 2000, vol. 189, pp. 79–90. https://doi.org/10.1006/jcat.1999.2703
Kim, J.C., Li, H.-X., Chen, C.-Y., and Davis, M.E., Microporous Mater., 1994, vol. 2, pp. 413–423. https://doi.org/10.1016/0927-6513(94)00008-5
Jacobs, P.A. and Uytterhoeven, J.B., J. Catal., 1977, vol. 50, pp. 109–114. https://doi.org/10.1016/0021-9517(77)90013-6
Hathaway, P.E. and Davis, M.E., J. Catal., 1989, vol. 116, pp. 263–278. https://doi.org/10.1016/0021-9517(89)90091-2
Hathaway, P.E. and Davis, M.E., J. Catal., 1989, vol. 116, pp. 279–284. https://doi.org/10.1016/0021-9517(89)90092-4
Cocepcion-Heydorn, P., Jia, C., Pfander, N., Karge, H.G., and Jentoft, F.C., J. Mol. Catal. A, 2000, vol. 162, pp. 227–246. https://doi.org/10.1016/S1381-1169(00)00292-2
Fu, Z-H. and Ono, Y., Catal. Lett., 1993, vol. 21, pp. 43–47. https://doi.org/10.1007/BF00767369
Wieland, W.S., Davis, R.J., and Garses, J.M., J. Catal., 1998, vol. 173, pp. 490–500. https://doi.org/10.1006/jcat.1997.1952
Borgna, A., Sepulveda, J., Magni, S.I., and Apesteguia, C.R., Appl. Catal. A: Gen., 2004, vol. 276, pp. 207–215. https://doi.org/10.1016/j.apcata.2004.08.007
Palomares, A.E., Eder-Marth, G., Rep, M., and Lercher, J.A., J. Catal., 1998, vol. 180, pp. 56–65. https://doi.org/10.1006/jcat.1998.2253
Sooknoi, T. and Dwyer, J., J. Mol. Catal. A, 2004, vol. 211, pp. 155–164. https://doi.org/10.1016/j.molcata.2003.10.017
Su, B.L. and Barthomeuf, D., Appl. Catal. A: Gen., 1995, vol. 124, pp. 73–80. https://doi.org/10.1016/0926-860X(94)00247-9
Ivanova, I.I., Pomakhina, E.B., Rebrov, A.I., Wang, W., Hunger, M., and Weitkamp, J., Kinet. Catal., 2003, vol. 44, pp. 701–709 https://doi.org/10.1023/A:1026158525990
Ponomareva, O.A., Shaposhnik, P.A., Belova, M.V., Kolozhvari, B.A., and Ivanova, I.I., Front. Chem. Sci. Eng., 2018, vol. 12, pp. 70–76. https://doi.org/10.1007/s11705-017-1694-3
Zeolites in Catalysis: Properties and Applications, RSC Catalysis Series, 2017.
Karge, H.G. and Beyer, H.K., Molecular Sieves. Science and Technology, 2002, vol. 3, pp. 43–201. https://doi.org/10.1007/3-540-69750-0_2
Sherry, H.S., J. Phys. Chem., 1966, vol. 70, pp. 1158–1168. https://doi.org/10.1021/j100876a031
Norby, P., Poshni, P.A., Gualtiery, A.F., Hanson, J.C., and Grey, C.P., J. Phys. Chem., 1998, vol. 102, pp. 839–856. https://doi.org/10.1021/jp9730398
Koller, H., Burger, B., Schneider, A.M., and Engelhart, G.W.J., Microporous Mater., 1995, vol. 5, pp. 219–232. https://doi.org/10.1016/0927-6513(95)00061-5
Gerzeliev, I.M., Ostroumova, V.A., Baskhanova, M.N., Saitov, Z.A., Temnikova, V.A., and Khusaimova, D.O., Petrol. Chem., 2017, vol. 57, pp. 1182–1185. https://doi.org/10.1134/S0965544117060147
Tamura, M., Shimizu, K., and Satsuma, A., Appl. Catal. A, 2012, vols. 433–434, pp. 135–145. http://dx.doi.org/10.1016/j.apcata.2012.05.008
Bordiga, S., Lamberti, C., Bonino, F., Travertd, A., and Thibault-Starzyk, F., Chem. Soc. Rev., 2015, vol. 44, pp. 7262–7343. https://doi.org/10.1039/C5CS00396B
Romero, M.D., Ovejero, G., Rodriguez, A., and Gomez, J.M., Microp. Mesopor. Mater., 2005, vol. 81, pp. 313–320. https://doi.org/10.1016/j.micromeso.2005.02.013
Funding
The NMR examination of the samples was performed with financial support from the Russian Science Foundation (RSF Grant no. 20-13-00203). The synthesis of the samples and the investigation of their physicochemical and catalytic properties were carried out within the State Program “Physical chemistry of surface adsorption and catalysis.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
I.I. Ivanova, a co-author, is the Chief Editor at the Sovremennye molekulyarnye sita (Advanced Molecular Sieves) Journal. The other co-authors declare no conflict of interest requiring disclosure in this article.
Rights and permissions
About this article
Cite this article
Ponomareva, O.A., Shaposhnik, P.A., Nazarova, V.I. et al. Effects of Ion Exchange Degree on the Physicochemical and Catalytic Properties of CsNaY. Pet. Chem. 62, 301–309 (2022). https://doi.org/10.1134/S0965544122010042
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544122010042