Abstract
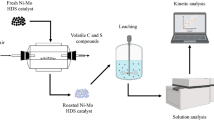
Composite catalysts containing molybdenum are mostly used for the purpose of hydrogenation in the oil refining industries. These treatment processes leave behind a large amount of used catalyst. These worn-out catalysts can be a secondary source of metal recovery for molybdenum and increase environmental awareness. In this paper, molybdenum recovery from worn-out catalysts has been investigated by leaching method in alkali medium (ammonia, NH3) after roasting process. The experimental data indicate that the roasting temperature and period, leaching temperature and duration are highly effective on the dissolution yield of molybdenum. The maximum dissolution rates of molybdenum (92.12%) was reached under optimum leaching conditions; roasting temperature, 600°C; roasting time, 120 min; particle size, +75–30 µm; liquid/solid ratio, 6 mL/g; ammonia concentration, 1 M; leaching temperature, 80°C; leaching time, 90 min and stirring speed, 400 r/min. Kinetic results indicate that the dissolution reactions of molybdenum is controlled by the liquid film diffusion mechanism. Activation energy value (Ea) of molybdenum was found to be as 10.89 kJ/mol.













Similar content being viewed by others
REFERENCES
Akcil, A.F., Vegliò, F., Ferella, F., Okudan, M.D., and Tuncuk, A., Waste Manage., 2015, vol. 45, p. 420. https://doi.org/10.1016/j.wasman.2015.07.007
Marafi, M. and Stanislaus, J. Hazard. Mater., 2003, vol. 101, no. 2, p. 123. https://doi.org/10.1016/S0304-3894(03)00145-6
Marafi, M. and Stanislaus, A., Resour. Conserv. Recycl., 2008, vol. 52, no. 6, p. 859. https://doi.org/10.1016/j.resconrec.2008.02.004
Marafi, M. and Stanislaus, A., Resour. Conserv. Recycl., 2008, vol. 53, no. 1–2, p. 1. https://doi.org/10.1016/j.resconrec.2008.08.005
Dufresne, P., Appl. Catal. A: Gen., 2007, vol. 322, p. 67. https://doi.org/10.1016/j.apcata.2007.01.013
Liu, C., Yu, Y., and Zhao, H., Fuel Process. Technol., 2005, vol. 86, no. 4, p. 449. https://doi.org/10.1016/j.fuproc.2004.05.002
Asghari, I., Mousavi, S.M., Amiri, F., and Tavassoli, S., J. Ind. Eng. Chem., 2013, vol. 19, no. 4, p. 1069. https://doi.org/10.1016/j.jiec.2012.12.005
Macaskie, L.E., Mikheenko, I.P., Yong, P., Deplanche, K., Murray, A.J., Paterson-Beedle, M., and Vaughan, D., Hydrometallurgy, 2010, vol. 104, nos. 3–4, p. 483. https://doi.org/10.1016/j.hydromet.2010.01.018
Pradhan, J.K. and Kumar, S., Waste Manage. Res., 2012, vol. 30, no. 11, p. 1151. https://doi.org/
Marafi, A., Fukase, S., Al-Marri, M., and Stanislaus, A., Energy Fuels, 2003, vol. 17, no. 3, p. 661. https://doi.org/10.1021/ef020177+
Arslanoğlu, H. and Yaraş, A., Petrol. Sci. Technol., 2019, vol. 37, no. 19, p. 2081. https://doi.org/10.1080/10916466.2019.1618867
Yaraş, A. and Arslanoğlu, H., Sep. Sci. Technol., 2020, vol. 55, no. 11, p. 2037. https://doi.org/10.1080/01496395.2019.1673412
Motaghed, M., Mousavi, S.M., Rastegar, S.O., and Shojaosadati, S.A., Bioresour. Technol., 2014, vol. 171, p. 401. https://doi.org/10.1016/j.biortech.2014.08.032
Mishra, D., Chaudhury, G.R., Kim, D.J., and Ahn, J.G., Hydrometallurgy, 2010, vol. 101, nos. 1–2, p. 35. https://doi.org/10.1016/j.hydromet.2009.11.016
Mohapatra, D.and Park, K.H., J. Environ. Sci. Heal. Part A, 2007, vol. 42, no. 4, p. 507. https://doi.org/10.1080/10934520601188409
Chaudhary, A.J., Donaldson, J.D., Boddington, S.C., and Grimes, S.M., Hydrometallurgy, 1993, vol. 34, no. 2, p. 137. https://doi.org/10.1016/0304-386X(93)90031-8
Ognyanova, A., Ozturk, A.T., De Michelis, I., Ferella, F., Taglieri, G., Akcil, A., and Vegliò, F., Hydrometallurgy, 2009, vol. 100, nos. 1–2, p. 20. https://doi.org/10.1016/j.hydromet.2009.09.009
Visaliev, M.Y., Shpirt, M.Y., Kadiev, K.M., Dvorkin, V.I., Magomadov, E.E., and Khadzhiev, S.N., Solid Fuel Chem., 2012, vol. 46, no. 2, p. 100. https://doi.org/10.3103/S0361521912020127
Arslanoğlu, H. and Yaraş, A., Petrol. Sci. Technol., 2019, vol. 37, no. 19, p. 2081. https://doi.org/10.1080/10916466.2019.1618867
Ferella, F., Ognyanova, A., De Michelis, I., Taglieri, G., and Vegliò, F., J. Hazard. Mater., 2011, vol. 192, no. 1, p. 176. https://doi.org/10.1016/j.jhazmat.2011.05.005
Yaraş, A. and Arslanoğlu, H., Can. Metall. Quart., 2018, vol. 57, no. 3, p. 319. https://doi.org/10.1080/00084433.2018.1473136
Arslanoğlu, H. and Yaraş, A., Trans. Indian Inst. Met., 2020, vol. 73, no. 3, p. 785. https://doi.org/10.1007/s12666-020-01896-x
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that there are no conflicts of interest regarding the publication of this paper.
Rights and permissions
About this article
Cite this article
Arslanoğlu, H. Selective Recovery of Molybdenum from Petroleum Industry Waste Spent Hydrodesulfurization Mo–Co–Ni/Al2O3 Catalyst in the Presence of Ammonia: Process Optimization and Kinetic Studies. Pet. Chem. 61, 198–205 (2021). https://doi.org/10.1134/S0965544121020043
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544121020043