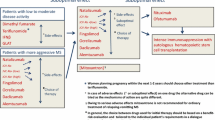
Abstract
Monoclonal antibodies are one of the fastest growing areas of specific therapy for cancer, infectious, autoimmune, and demyelinating diseases. The main targets of monoclonal antibodies in autoimmune and demyelinating diseases are T and B lymphocytes, cytokines, complement, and adhesion molecules. In the field of neurodegenerative diseases, the largest number of studies has been carried out in patients with Alzheimer’s disease, in which the target for monoclonal antibodies is pathologically altered beta-amyloid protein that accumulates in the brain parenchyma. This review presents new therapeutic approaches to the use of antibody fragments, with an emphasis on their new class, nanobodies. Special attention is paid to new anti-CD20 and anti-CD19 monoclonal antibody drugs in multiple sclerosis (ocrelizumab, ofatumumab) and neuromyelitis optica (inebilizumab). Ocrelizumab is the first medication that proved to be effective in primary progressive multiple sclerosis. There have been significant advances in the treatment of neuromyelitis optica: the first multicenter study of Inebilizumab (MEDI-551) is currently underway.


Similar content being viewed by others
REFERENCES
Gajofatto, A. and Turatti, M., Investigational immunosuppressants in early-stage clinical trials for the treatment of multiple sclerosis, Expert Opin. Invest. Drugs, 2018, vol. 27, no. 3, p. 273. https://doi.org/10.1080/13543784.2018.1442437
Montalban, X., Hauser, S.L., Kappos, L., et al., Ocrelizumab versus placebo in primary progressive multiple sclerosis, N. Engl. J. Med., 2017, vol. 376, no. 3, p. 209. https://doi.org/10.1056/NEJMoa1606468
Forsthuber, T.G., Cimbora, D.M., Ratchford, J.N., et al., B cell-based therapies in CNS autoimmunity: differentiating CD19 and CD20 as therapeutic targets, Ther. Adv. Neurol. Disord., 2018, vol. 11, p. 1756286418761697. https://doi.org/10.1177/1756286418761697
Paul, F., Murphy, O., Pardo, S., and Levy, M., Investigational drugs in development to prevent neuromyelitis optica relapses, Expert Opin. Invest. Drugs, 2018, vol. 27, no. 3, p. 265. https://doi.org/10.1080/13543784.2018.1443077
Arumugakani, G., Stephenson, S.J., Newton, D.J., et al., Early emergence of CD19-negative human antibody-secreting cells at the plasmablast to plasma cell transition, J. Immunol., 2017, vol. 198, no. 12, p. 4618.https://doi.org/10.4049/jimmunol.1501761
van Dyck, C.H., Anti-amyloid-β monoclonal antibodies for Alzheimer’s disease: pitfalls and promise, Biol. Psychiatry, 2017, vol. 83, no. 4, p. 311.https://doi.org/10.1016/j.biopsych.2017.08.010
Hamers-Casterman, C., Atarhouch, T., Muylder-mans, S., et al., Naturally occurring antibodies devoid of light chains, Nature, 1993, vol. 363, no. 6428, p. 446. https://doi.org/10.1038/363446a0
Rissiek, B., Koch-Nolte, F., and Magnus, T., Nanobodies as modulators of inflammation: potential applications for acute brain injury, Front. Cell. Neurosci., 2014, vol. 8, p. 344. https://doi.org/10.3389/fncel.2014.00344
Peyvandi, F., Scully, M., Kremer, Hovinga, J.A., et al., Caplacizumab reduces the frequency of major thromboembolic events, exacerbations and death in patients with acquired thrombotic thrombocytopenic purpura, J. Thromb. Haemost., 2017, vol. 15, no. 7, p. 1448. https://doi.org/10.1111/jth.13716
Butler, D.C., Joshi, S.N., De Genst, E., et al., Bifunctional anti-non-amyloid component α-synuclein nanobodies are protective in situ, PloS One, 2016, vol. 11, no. 11. e0165964. https://doi.org/10.1371/journal.pone.0165964
Muyldermans, S., Nanobodies: natural single-domain antibodies, Annu. Rev. Biochem., 2013, vol. 82, p. 775. https://doi.org/10.1146/annurev-biochem-063011-092449
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Deryabina
Rights and permissions
About this article
Cite this article
Zakharova, M.N. Monoclonal Antibodies in Neurology: Current State and Future Development. Hum Physiol 48, 932–937 (2022). https://doi.org/10.1134/S0362119722080072
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0362119722080072