Abstract
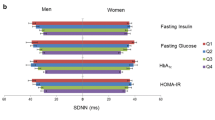
Currently, monitoring of blood glucose level (BGL) is constrained by the invasive nature of BGL measures. We investigated heart rate variability (HRV) parameters as potential non-invasive markers of BGL. Healthy volunteers (n = 25; aged 27 ± 9 years) uninhibited by regular medications or chronic illness were recruited for this study. BGL and HRV were assessed during fasting (9:00 am), postprandial (12:00 pm), and postabsorptive (3:00 pm) periods using self-monitoring of blood glucose techniques and ten-minute electrocardiogram, respectively. Frequency-domain HRV measures, which estimate contributions of sympathetic and parasympathetic systems to autonomic modulation of the heart, were correlated against BGL data with the following significant (p < 0.05) findings. The change in BGL from fasting to postprandial levels was negatively correlated with fasting low frequency (LF) power and total power (TP). Postprandial BGL was negatively associated with fasting LF and TP, as well as with postprandial LF, high frequency (HF), and TP. The change in BGL from postprandial to postabsorptive levels was positively correlated with fasting LF power, as well as with postprandial LF, HF, and TP. Frequency-domain HRV parameters may be useful in predicting the magnitude and direction of acute fluctuations in BGL, and further research could develop them as non-invasive markers of BGL.
Similar content being viewed by others
REFERENCES
Cho, N., Shaw, J., Karuranga, S., et al., IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045, Diabetes Res. Clin. Pract., 2018, vol. 138, p. 271.
American Diabetes Association, Diagnosis and classification of diabetes mellitus, Diabetes Care, 2010, vol. 33, no. 1, p. 62.
Andersen, L.B., Mota, J., and Di Pietro, L., Update on the global pandemic of physical inactivity, Lancet, 2016, vol. 388, no. 1005, p. 1255.
Carrera-Bastos, P., Fontes-Villalba, M., O’Keefe, J.H., et al., The western diet and lifestyle and diseases of civilization, Res. Rep. Clin. Cardiol., 2011, vol. 2, p. 15.
Inzucchi, S.E., Bergenstal, R.M., Buse, J.B., et al., Management of hyperglycemia in type 2 diabetes: a patient-centered approach, Diabetes Care, 2012, vol. 35, no. 6, p. 1364.
Brownlee, M., The pathobiology of diabetic complications a unifying mechanism, Diabetes, 2005, vol. 54, no. 6, p. 1615.
Pop-Busui, R., Evans, G.W., Gerstein, H.C., et al., Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, Diabetes Care, 2010, vol. 33, no. 7, p. 1578.
Vinik, A.I., Erbas, T., and Casellini, C.M., Diabetic cardiac autonomic neuropathy, inflammation and cardiovascular disease, J. Diabetes Invest, 2013, vol. 4, no. 1, p. 4.
Wulsin, L.R., Horn, P.S., Perry, J.L., et al., Autonomic imbalance as a predictor of metabolic risks, cardiovascular disease, diabetes, and mortality, J. Clin. Endocrinol. Metab., 2015, vol. 100, no. 6, p. 2443.
Ray, K.K., Seshasai, S.R.K., Wijesuriya, S., et al., Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials, Lancet, 2009, vol. 373, no. 9677, p. 1765.
Shima, K., Komatsu, M., Kawahara, K., et al., Stringent glycaemic control prolongs survival in diabetic patients with end-stage renal disease on haemodialysis, Nephrology, 2010, vol. 15, no. 6, p. 632.
Beagley, J., Guariguata, L., Weil, C., and Motala, A.A., Global estimates of undiagnosed diabetes in adults, Diabetes Res. Clin. Pract., 2014, vol. 103, no. 2, p. 150.
Michaelides, C., Daja, M., Peterson, D., and Conroy, D., An HbA1c mapping tool helps identify where interventions and strategies for change need to be targeted, The Mapping Glycaemic Control Across Australia (MGCAA) project, 2012. http://www.glycomate.com/changingdiabetes/.
Vashist, S.K., Non-invasive glucose monitoring technology in diabetes management: a review, Anal. Chim. Acta, 2012, vol. 750, p. 16.
Halldorsdottir, S., Warchal-Windham, M.E., Wallace, J.F., et al., Accuracy evaluation of five blood glucose monitoring systems: the North American Comparator Trial, J. Diabetes Sci. Technol., 2013, vol. 7, no. 5, p. 1294.
Tack, C., Pohlmeier, H., Behnke, T., et al., Accuracy evaluation of five blood glucose monitoring systems obtained from the pharmacy: a European multicenter study with 453 subjects, Diabetes Technol. Ther., 2012, vol. 14, no. 4, p. 330.
Ong, W.M., Chua, S.S., and Ng, C.J., Barriers and facilitators to self-monitoring of blood glucose in people with type 2 diabetes using insulin: a qualitative study, Patient Preference Adherence, 2014, vol. 8, no. 2014, p. 237.
Bandodkar, A.J. and Wang, J., Non-invasive wearable electrochemical sensors: a review, Trends Biotechnol., 2014, vol. 32, no. 7, p. 363.
Do Amaral, C.E.F. and Wolf, B., Current development in non-invasive glucose monitoring, Med. Eng. Phys., 2008, vol. 30, no. 5, p. 541.
Kleiger, R.E., Miller, J.P., Bigger, J.T., and Moss, A.J., Decreased heart rate variability and its association with increased mortality after acute myocardial infarction, Am. J. Cardiol., 1987, vol. 59, no. 4, p. 256.
Jaiswal, M., Urbina, E.M., Wadwa, R.P., et al., Reduced heart rate variability among youth with type 1 diabetes the SEARCH CVD study, Diabetes Care, 2013, vol. 36, no. 1, p. 157.
Javorka, M., Javorková, J., Tonhajzerová, I., et al., Heart rate variability in young patients with diabetes mellitus and healthy subjects explored by Poincaré and sequence plots, Clin. Physiol. Funct. Imaging, 2005, vol. 25, no. 2, p. 119.
Kudat, H., Akkaya, V., Sozen, A., et al., Heart rate variability in diabetes patients, J. Int. Med. Res., 2006, vol. 34, no. 3, p. 291.
Mirza, M. and Lakshmi, A., A comparative study of heart rate variability in diabetic subjects and normal subjects, Int. J. Biomed. Adv. Res., 2012, vol. 3, no. 8, p. 640.
Zygmunt, A. and Stanczyk, J., Methods of evaluation of autonomic nervous system function, Arch. Med. Sci., 2010, vol. 6, no. 1, p. 11.
Buccelletti, E., Gilardi, E., Scaini, E., et al., Heart rate variability and myocardial infarction: systematic literature review and metanalysis, Eur. Rev. Med. Pharmacol. Sci., 2009, vol. 13, no. 4, p. 299.
Huikuri, H.V. and Stein, P.K., Clinical application of heart rate variability after acute myocardial infarction, Front. Physiol., 2012, vol. 3, p. 41.
Thayer, J.F., Yamamoto, S.S., and Brosschot, J.F., The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors, Int. J. Cardiol., 2010, vol. 141, no. 2, p.122.
Spallone, V., Update on the impact, diagnosis and management of cardiovascular autonomic neuropathy in diabetes: what is defined, what is new, and what is unmet, Diabetes Metab. J., 2019, vol. 43, no. 1, p. 3.
Jarczok, M.N., Li, J., Mauss, D., et al., Heart rate variability is associated with glycemic status after controlling for components of the metabolic syndrome, Int. J. Cardiol., 2013, vol. 167, no. 3, p. 855.
Klimontov, V.V., Myakina, N.E., and Tyan, N.V., Heart rate variability is associated with interstitial glucose fluctuations in type 2 diabetic women treated with insulin, SpringerPlus, 2016, vol. 5, no. 337, p. 1.
Lutfi, M.F. and Elhakeem, R.F., Effect of fasting blood glucose level on heart rate variability of healthy young adults, PLoS One, 2016, vol. 11, no. 7, p. e0159820.
Rothberg, L.J., Lees, T., Clifton-Bligh, R., and Lal, S., Association between heart rate variability measures and blood glucose levels: implications for noninvasive glucose monitoring for diabetes, Diabetes Technol. Ther., 2016, vol. 18, no. 6, p. 366.
Singh, J.P., Larson, M.G., O’Donnell, C.J., et al., Association of hyperglycemia with reduced heart rate variability (the Framingham Heart Study), Am. J. Cardiol., 2000, vol. 86, no. 3, p. 309.
Stein, P., Barzilay, J., Domitrovich, P., et al., The relationship of heart rate and heart rate variability to non-diabetic fasting glucose levels and the metabolic syndrome: the Cardiovascular Health Study, Diabetes Med., 2007, vol. 24, no. 8, p. 855.
Weissman, A., Lowenstein, L., Peleg, A., et al., Power spectral analysis of heart rate variability during the 100-g oral glucose tolerance test in pregnant women, Diabetes Care, 2006, vol. 29, no. 3, p. 571.
Kalopita, S., Liatis, S., Thomakos, P., et al., Relationship between autonomic nervous system function and continuous interstitial glucose measurement in patients with type 2 diabetes, J. Diabetes Res., 2014, vol. 2014, art. ID 835392.
Kanaley, J.A., Baynard, T., Franklin, R.M., et al., The effects of a glucose load and sympathetic challenge on autonomic function in obese women with and without type 2 diabetes mellitus, Metabolism, 2007, vol. 56, no. 6, p. 778.
Avignon, A., Radauceanu, A., and Monnier, L., Nonfasting plasma glucose is a better marker of diabetic control than fasting plasma glucose in type 2 diabetes, Diabetes Care, 1997, vol. 20, no. 12, p. 1822.
Monnier, L., Lapinski, H., and Colette, C., Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA1c, Diabetes Care, 2003, vol. 26, no. 3, p. 881.
Kilinc, O., Cincin, A., Pehlivan, A., et al., Assessment of time and frequency domain parameters of heart rate variability and interictal cardiac rhythm abnormalities in drug-naïve patients with idiopathic generalized epilepsy, J. Epilepsy Res. 2016, vol. 6, no. 1, p. 22.
Wolever, T.M., Chiasson, J.-L., Csima, A., et al., Variation of postprandial plasma glucose, palatability, and symptoms associated with a standardized mixed test meal versus 75 g oral glucose, Diabetes Care, 1998, vol. 21, no. 3, p. 336.
Freckmann, G., Schmid, C., Baumstark, A., et al., System accuracy evaluation of 43 blood glucose monitoring systems for self-monitoring of blood glucose according to DIN EN ISO 15197, J. Diabetes Sci. Tech-nol., 2012, vol. 6, no. 5, p. 1060.
Morgan, L.M., Shi, J.-W., Hampton, S.M., and Frost, G., Effect of meal timing and glycaemic index on glucose control and insulin secretion in healthy volunteers, Br. J. Nutr., 2012, vol. 108, no. 7, p.1286.
WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy, Geneva: World Health Org., 2010.
Pickering, T.G., Hall, J.E., Appel, L.J., et al., Recommendations for blood pressure measurement in humans and experimental animals, Circulation, 2005, vol. 111, no. 5, p. 697.
Melzig, C.A., Weike, A.I., Hamm, A.O., and Thayer, J.F., Individual differences in fear-potentiated startle as a function of resting heart rate variability: implications for panic disorder, Int. J. Psychophysiol., 2009, vol. 71, no. 2, p. 109.
McNames, J. and Aboy, M., Reliability and accuracy of heart rate variability metrics versus ECG segment duration, Med. Biol. Eng. Comput., 2006, vol. 44, no. 9, p. 747.
Pichon, A., Roulaud, M., Antoine-Jonville, S., et al., Spectral analysis of heart rate variability: interchangeability between autoregressive analysis and fast Fourier transform, J. Electrocardiol., 2006, vol. 39, no. 1, p. 31.
Tarvainen, M.P., Niskanen, J.-P., Lipponen, J.A., et al., Kubios HRV—heart rate variability analysis software, Comput. Methods Progr. Biomed., 2014, vol. 113, no. 1, p. 210.
Penčić-Popović, B., Ćelić, V., Ćosić, Z., et al., Heart rate variability and increased risk for developing type 2 diabetes mellitus, Vojnosanit. Pregl., 2014, vol. 71, no. 12, p. 1109.
Sztajzel, J., Heart rate variability: a noninvasive electrocardiographic method to measure the autonomic nervous system, Swiss Med. Wkly., 2004, vol. 134, p. 514.
Delaney, J.P.A. and Brodie, D.A., Effects of short-term psychological stress on the time and frequency domains of heart-rate variability, Percept. Mot. Skills, 2000, vol. 91, no. 2, p. 515.
Kobayashi, H., Park, B.-J., and Miyazaki, Y., Normative references of heart rate variability and salivary alpha-amylase in a healthy young male population, J. Physiol. Anthropol., 2012, vol. 31, no. 1, p. 9.
Ramaekers, D., Ector, H., Aubert, A.E., et al., Heart rate variability and heart rate in healthy volunteers. Is the female autonomic nervous system cardioprotective? Eur. Heart J., 1998, vol. 19, no. 9, p. 1334.
Benichou, T., Pereira, B., Mermillod, M., et al., Heart rate variability in type 2 diabetes mellitus: a systematic review and meta–analysis, PLoS One, 2018, vol. 13, no. 4, p. e0195166.
França da Silva, A.K., da Costa de Rezende Barbosa, M.P., Vanderlei, F.M., et al., Application of heart rate variability in diagnosis and prognosis of individuals with diabetes mellitus: systematic review, Ann. Noninvasive Electrocardiol., 2016, vol. 21, no. 3, p. 223.
Valensi, P., Pariès, J., Attali, J.R., et al., Cardiac autonomic neuropathy in diabetic patients: influence of diabetes duration, obesity, and microangiopathic complications—the French multicenter study, Metabolism, 2003, vol. 52, no. 7, p. 815.
Wheeler, T. and Watkins, P., Cardiac denervation in diabetes, Br. Med. J., 1973, vol. 4, no. 5892, p. 584.
Lefrandt, J.D., Mulder, M.C., Bosma, E., et al., Inverse relationship between blood glucose and autonomic function in healthy subjects, Diabetes Care, 2000, vol. 23, no. 12, p. 1862.
D’alessio, D.A., Kieffer, T.J., Taborsky, G.J., Jr., and Havel, P.J., Activation of the parasympathetic nervous system is necessary for normal meal-induced insulin secretion in rhesus macaques, J. Clin. Endocrinol. M-etab., 2001, vol. 86, no. 3, p. 1253.
Nederkoorn, C., Smulders, F., and Jansen, A., Cephalic phase responses, craving and food intake in normal subjects, Appetit, 2000, vol. 35, no. 1, p. 45.
Tillil, H., Shapiro, E.T., Miller, M.A., et al., Dose-dependent effects of oral and intravenous glucose on insulin secretion and clearance in normal humans, Am. J. Physiol., 1988, vol. 254, no. 3, p. E349.
Bowes, S., Hennessy, T., Umpleby, A., et al., Measurement of glucose metabolism and insulin secretion during normal pregnancy and pregnancy complicated by gestational diabetes, Diabetologia, 1996, vol. 39, no. 8, p. 976.
Heathers, J.A., Everything Hertz: methodological issues in short-term frequency-domain HRV, Front. Physiol., 2014, vol. 5, p. 177.
Massin, M.M., Maeyns, K., Withofs, N., et al., Circadian rhythm of heart rate and heart rate variability, Arch. Dis. Child., 2000, vol. 83, no. 2, p. 179.
Jensen-Urstad, K., Storck, N., Bouvier, F., et al., Heart rate variability in healthy subjects is related to age and gender, Acta Physiol. Scand., 1997, vol. 160, no. 3, p. 235.
O’Brien, I., O’Hare, P., and Corrall, R., Heart rate variability in healthy subjects: effect of age and the derivation of normal ranges for tests of autonomic function, Br. Heart J., 1986, vol. 55, no. 4, p. 348.
Ding, S. and Schumacher, M., Sensor monitoring of physical activity to improve glucose management in diabetic patients: a review, Sensors, 2016, vol. 16, no. 4, p. 589.
Cichosz, S.L., Frystyk, J., Hejlesen, O.K., et al., A novel algorithm for prediction and detection of hypoglycemia based on continuous glucose monitoring and heart rate variability in patients with type 1 diabetes, J. Diabetes Sci. Technol., 2014, vol. 8, no. 4, p. 731.
Schäfer, A. and Vagedes, J., How accurate is pulse rate variability as an estimate of heart rate variability?: A review on studies comparing photoplethysmographic technology with an electrocardiogram, Int. J. Cardiol., 2013, vol. 166, no. 1, p. 15.
ACKNOWLEDGMENTS
The authors would like to acknowledge the Neuroscience Research Unit of the University of Technology Sydney for providing the equipment and facilities necessary to complete this study.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, Roderick Clifton-Bligh, Ann M Simpson, Najah Nassif and Sara Lal; Data curation, Ty Lees; Formal analysis, Jaymen L Elliott; Investigation, Luke R Jarman; Methodology, Luke R Jarman, Jaymen L Elliott and Sara Lal; Resources, Ty Lees; Supervision, Roderick Clifton-Bligh, Ann M Simpson, Najah Nassif and Sara Lal; Writing—original draft, Luke R Jarman; Writing—review & editing, Ty Lees, Jaymen Elliott, Roderick Clifton-Bligh, Ann M Simpson, Najah Nassif and Sara Lal.
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Jarman, L.R., Elliott, J.L., Lees, T. et al. Heart Rate Variability as a Potential Non-invasive Marker of Blood Glucose Level. Hum Physiol 47, 209–218 (2021). https://doi.org/10.1134/S0362119721020031
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0362119721020031