Abstract
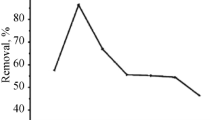
In this study, ability of Nymphaea alba (White Water lily) leaves to remove Ni2+ from aqueous solution was studied in the batch system. HCl, MgCl2, and CaCl2 chemical modification of N. alba leaves showed increasing sorption capacity of Ni2+ (24 mg/g; pH 6.5). Removal percentage of Ni2+ increased by adding more biomass. Increasing Ni2+ concentration up to 210 mg/L and temperature up to 35°C caused higher biosorption capacity. However, higher Ni2+ concentrations and rising temperature had vice versa effect. The optimum agitation time was 90 min. The prolongation of the agitation period had no considerable effect on the biosorption capacity. The kinetics study of modified N. alba leaves revealed that Ni2+ sorption follows pseudo-second order kinetic model. Estimated parameters, the rate constant of second order biosorption (K 2) and biosorption capacity (q e ), for this model at T = 293 K were 0.225 g/mg min and 17.42 mg/g, respectively. The experimental results were correlated reasonably well with Langmuir model (q max = 118 mg/g; b = 0.022 L/mg). The changes in Gibb’s free energy (ΔG°), enthalpy (ΔH°) and entropy (ΔS°) of Ni2+ sorption by modified N. alba leaves were estimated as–1.726, 0.208, and 0.0018 KJ/mol K, respectively. Thermodynamic parameters revealed spontaneous, endothermic, and irreversible nature for Ni+2 sorption. XRF and FT–IR analysis results showed that N. alba leaves had different functional carboxyl and sulfhydryl groups responsible for the sorption process.
Similar content being viewed by others
References
Abia, A., Didi, O., and Asuquo, E., Modeling of Cd+2 sorption kinetices from aqeous solutions on to some thiolated agricultural waste adsorbents, J. Appl. Sci., 2006, vol. 6, no.12, pp. 2549–2556.
Ahmady-Asbchin, S., Mohammadi, M., Bahrami, A., Loueimonfared, A., and Jafari, N., Batch studies on the removal of Ni (II) from aqueous solution by Azolla filiculoides, Afr. J. Biotechnol., 2011, vol.10, no. 38, pp. 7427–7431.
Babalola, J., Babarinde, N., Oninla, V., and Popoola, O, Kinetics, equilibrium, and thermodynamics studies of the biosorption of lead (II) and chromium (III) by Basella alba L, The Pacific J. Sci. and Technol., 2008, vol. 9, pp. 610–620.
Chen, C. and Wang, X., Adsorption of Ni (II) from aqueous solution using oxidized multiwall carbon nanotubes, Ind. Eng. Chem. Res., 2006, vol. 45, pp. 9144–9149.
Cruz, C.V., Costa, A.A., Henriques, C.A., and Luna, A.S., Kinetic modeling and equilibrium studies during cadmium biosorption by dead Sargassum sp. biomass, Bioresour. Technol., 2004, vol. 91, pp. 249–257.
Dave, P., Subrahmanyarn, N., and Sharna, S., Kinetics and thermodynamics of copper ions removal from aqueos solution by use of activated characoal, Indian J. Chem. Technol., 2009, vol.16, pp. 234–239.
Dhir, B. and Kumar, R., Adsorption of heavy metals by salvinia biomass and agricultural residues, Int. J. Environ. Res., Summer, 2010, vol. 4, no. 3, pp. 427–432.
Gnaji, M., Khosravi, M., and Rakhshaee, R., Biosorption of Pb, Cd, Cu, and Zn from the wastewater by treated Azolla filiculoides with H2O2/MgCl2, Int. J. Environ. Sci. Technol., 2005, vol. 1, no. 4, pp. 265–271.
Herrero, R., Cordero, B., Lodeiro, P., Rey-Castro, C., and Sastre de Vicente, M.E., Interactions of cadmium (II) and protons with dead biomass of marine algae Fucus sp., Mar. Chem., 2006, vol. 99, pp. 106–116.
Igwe, J. and Abia, A., A bioseparation process for removing heavy metals from waste water using biosorbents, Afr. J. Biotechnol., 2006, vol. 5, no. 12, pp. 1167–1179.
Jalali, R., Ghafourian, H., Asef, D., Davarpanah, S., and Sepehr, S., Removal and recovery of lead using nonliving, J. Hazard. Mater., 2002, vol. 92, pp. 253–262.
Lagergren, S., Zur Theorie der sogenannten adsorption geloster stöffe. Kungliga Sevenska Vetenskapsakademiens, Handlinger, 1898, vol. 24, pp. 1–39.
Li, Y.H., Di, Z., Ding, J., Wu, D., Luan, Z., and Zhu, Y., Adsorption thermodynamic, kinetic and desorption studies of Pb2+ on carbon nanotubes, Water Research, 2005, vol. 39, pp. 605–609.
Lodeiro, P., Barriada, J., Herrero, R., and Sastre de Vicente, M., The marine macroalga Cystoseira baccata as biosorbent for cadmium (II) and lead (II) removal: Kinetic and equilibrium studies, Environ. Pollution, 2006, vol. 142, pp. 264–273.
Lu, C., Chiu, H., and Liu, C., Removal of zinc (II) from aqueous solution by purified carbon nanotubes: kinetics and equilibrium studies, Ind. Eng. Chem. Res., 2006, vol. 45, pp. 2850–2855.
Ofer, R., Yerachmiel, A., and Shmuel, Y., Marine macroalgae as biosorbents for cadmium and nickel in water, Water Environ. Res., 2003, vol. 75, no. 3, pp. 246–253.
Rosa, G. de la., Reynel-Avila, H., Bonilla-Petr, A., and Martínez-Hernández, A., Recycling poultry feathers for pb removal from wastewater: kinetic and equilibrium studies, World Acad. Sci., Eng. Technol., 2008, vol. 47, pp. 394–402.
Saygideger, S., Gulnaz, O., Salih Istifli, E., and Yucel, N., Adsorption of Cd (II), Cu (II) and Ni (II) ions by Lemna minor L.: Effect of physicochemical environment, J. Hazard. Mater., 2005, vol. B 126, pp. 96–104.
Silverstein, R., Bssler, G., Morrill, T., and Wiley, T. Spectrometric identification of organic compounds, N. Y.: J. Wiley and Sons, 1991.
Venkateswarlu, P., Vijaya Durga, G., Chitti Babu, N., and Venkateswara Rao, M., Biosorption of Zn (II) from an aqueous solution by Erythrina variegata orientalis leaf powder, Int. J. Phys. Sci., 2008, vol. 3, no. 9, pp. 197–204.
Vijayaraghavan, K., Palanivelu, K., and Velan, M., Biosorption of copper (II) and cobalt (II) from aqueous solutions by crab shell particles, Bioresour. Technol., 2006, vol. 97, pp. 1411–1419.
WHO, Guidelines for Drinking-Water Quality, 4th Edition, 2011.
Xin Sheng, P., Yen-Peng, T., Chen, J.P., and Hong, L., Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: characterization of biosorptive capacity and investigation of mechanisms, J. Colloid Interface Sci., 2004, vol. 275, pp. 131–141.
Xin, H., Nai-yun, G., and Qiao-li, Z., Thermodynamics and kinetics of cadmium adsorption onto oxidized granular activated carbon, J. Environ. Sci., 2007, vol. 19, pp. 1287–1292.
Yan, G.Y. and Viraraghavan, T., Heavy metal removal in a biosorption column by immobilized M. rouxii biomass, Bioresour. Technol., 2001, vol. 78, pp. 243–249.
Yang, L. and Chen, J.P., Biosorption of hexavalent chromium onto raw and chemically modified Sargassum sp., Bioresour. Technol., 2008, vol. 99, pp. 297–307.
Zafar, M.N., Nadeem, R., and Hanif, M., Biosorption of nickel from protonated rice bran, J. Hazard. Mater., 2006, vol. 143, pp. 478–485.
Özer A., Özer, D., and Özer, A., The adsorption of copper (II) ions on to dehydrated wheat bran (DWB) determination of the equilibrium and thermodynamic parameters, Process Biochemistry, 2004, vol. 39, pp. 2183–2191.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Zahedi, R., Dabbagh, R., Ghafourian, H. et al. Nickel removal by Nymphaea alba leaves and effect of leaves treatment on the sorption capacity: A kinetic and thermodynamic study. Water Resour 42, 690–698 (2015). https://doi.org/10.1134/S0097807815050152
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0097807815050152