Abstract
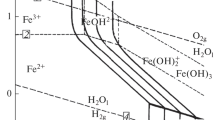
Foreign and domestic software products designed for quantitative description of the flow accelerated corrosion (FAC) rate and modeling erosion–corrosion use physicochemical computational models based on the assumption that the oxide film that protects the surface of steel consists of magnetite. Investigations of the phase composition and structural characteristics of oxide films on the inner surfaces of pipelines and equipment of coolant-circulation circuits of the NPP power-generating units with RBMK high-power channel-type reactors or the third circuits of the BN-800 fast reactors in which correction-free or oxygenated water chemistry conditions are implemented have revealed the presence on the steels' surface of not only magnetite but also other iron oxide forms. At present, the presence of the forms of iron oxides and hydroxides other than magnetite is considered in the computational modeling of the FAC by the introduction of empirical dependences. In the work, thermodynamic analysis of the existence forms of solid iron oxide and hydroxide phases in contact with the aqueous coolant in neutral oxygen-containing solutions has been made for temperatures of 25–200°C. To solve the problem set, the method of minimization of free energies of the system was used that allows one to determine which of the solid phases—iron oxides or hydroxides—will be thermodynamically stable in aqueous process media. On the assumption that the free energy of a system is minimal in the equilibrium state, the method allows determining the composition of the solid phase that is thermodynamically stable under the given conditions. The dependence of the phase composition of the oxide film on the temperature and chemical composition of the aqueous medium in contact with the oxide in question is shown. It is suggested to use the calculated results for refining the physicochemical models of the dependence of the FAC on the temperature for estimation of changes in the solubility of the iron oxides on the temperature and the thermodynamic stability regions of the basic phases in which iron oxides exist in the presence of oxygen and hydrogen.


Similar content being viewed by others
Notes
Here and below, the subscript “cr” denotes the crystalline state of the substance.
REFERENCES
N. E. Heitman and W. Kastner, “Erosionskorrosion in Wasser-Dampfkreislaufen — Ursachen und Gegenmassnamen,” VGB Kraftwerktech. 62, 211–219 (1982).
V. M. Sedov, P. G. Krutikov, E. A. Konstantinov, V. A. Shishkunov, and A. A. Afanas’ev, “Mössbauer spectroscopy in the phase-composition analysis of corrosion deposits in a nuclear power station,” Sov. At. Energy (Engl. Transl.) 46, 396–399 (1980).
J. A. Sawicki and M. E. Brett, “Mossbauer study of corrosion products from a CANDU secondary system,” Nucl. Instrum. Methods Phys. Res. B76, 254–257 (1993).
R. B. Dooley and V. K. Chexal, “Flow-accelerated corrosion of pressure vessels in fossil plants,” Int. J. Pressure Vessels Piping. 77, 85–90 (2000). https://doi.org/10.1016/S0308-0161(99)00087-3
T. Kh. Margulova, V. A. Mamet, and V. F. Tyapkov, “The content of iron oxides in the cycle of a single-circuit nuclear power plant,” Teploenergetika, No. 9, 62 (1981).
G. Bohnsack, “Zum Verstandnis der Schikkor-Reaction,” Mitt. Ver. Grosskesselbesitzer 51, 61–75 (1971).
O. I. Martynova, T. I. Petrova, and N. L. Kharitonova, “On the mechanism of formation of a protective oxide layer on the surface of carbon steel in a neutral-oxidative water chemistry,” Teploenergetika, No. 9, 15–19 (1982).
L. N. Moskvin, A. A. Efimov, S. B. Tomilov, E. P. Bredikhina, and A. I. Gorshkov, “Study of the interaction of aqueous solutions of hydrogen peroxide with pearlitic steels,” Teploenergetika, No. 6, 9–13 (1980).
L. N. Moskvin, “Studying the mechanism of formation of protective oxide films on the surface of pearlitic steels upon their contact with aqueous solutions of hydrogen peroxide,” Teploenergetika, No. 3, 52–55 (1981).
G. Bohnsack, “Chemische Grundlagen der Enteisenung,” Jahrb. Wasserchem. Wasserreinigungstech. 56, 284–296 (1976).
Yu. V. Shvarov, “Calculation of the equilibrium composition in a multicomponent heterogeneous system,” Dokl. Akad. Nauk SSSR 257, 1217–1226 (1981).
I. K. Karpov, A. I. Kiselev, and F. A. Letnikov, Computer Simulation of Natural Mineral Formation (Nedra, Moscow, 1976) [in Russian].
“Chemical thermodynamics of iron. Part. 1. NEA no. 6355,” in OECD Nuclear Energy Agency Data Bank, Ed. by J. Perrone (OECD, Paris, 2013). https://www. oecd-nea.org/dbtdb/pubs/6355-vol13a-iron.pdf
G. Bohnsack, Die Berechnung der Magnetitloslichkeit aus Thermodynamischen Daten (IAPS, 1985).
L. de Miranda, The Electrochemical Aspects of Atmospheric Corrosion Wearing of Steels. RT221 (Cebelcors Rapport Technique, 1974).
D. Langmir, Geological Survey Research.Chapter B:Geological Surrey Professional Paper (1969).
W. Feitknecht and P. Schindler, “Loeslichkeitskonstanten von Hydroxidfaellungen,” J. Pure Appl. Chem. 6, 130–190 (1963).
T. Misawa, “The thermodynamic consideration for Fe–H2O system at 25°C,” Corros. Sci. 13, 659–676 (1973). https://doi.org/10.1016/S0010-938X(73)80037-X
M. Pourbaix and X. Z. Yang, Chemical and Electrochemical Equilibria in the Presence of Gaseous Phase. Part 6. Oxygen–Hydrogen–Iron (Cebelcors Rapport Technique, 1981).
C. Criss and J. W. Cobble, “The thermodynamic properties of high temperature aqueous solutions. IV. Entropies of the ions up to 200 and the correspondence principle,” J. Am. Chem. Soc. 86, 5385–5390 (1964). https://doi.org/10.1021/ja01078a003
C. Criss and J. W. Cobble, “The thermodynamic properties of high temperature aqueous solutions. V. The calculation of ionic heat capacities up to 200. Entropies and heat capacities above 200,” J. Am. Chem. Soc. 86, 5390–5393 (1964). https://doi.org/10.1021/ja01078a004
Guideline on the Henry’s Constant and Vapor-Liquid Distribution Constant for Gases in H2O and D2O at High Temperatures (The International Association for the Properties of Water and Steam, 2004).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by O. Lotova
Rights and permissions
About this article
Cite this article
Kharitonova, N.L., Tyapkov, V.F. Thermodynamic Analysis of the Existing Forms of Iron Oxides in Neutral Oxygen-Containing Aqueous Media of the NPP Power-Generating Units. Therm. Eng. 67, 17–24 (2020). https://doi.org/10.1134/S0040601520010036
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040601520010036