Abstract
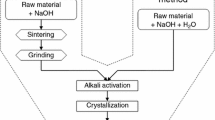
The faujasite and chabazite zeolites with 100% crystallinity and phase purity of potential practical importance was first obtained based on the mineral raw materials of the Nakhchivan Kyukyuchai deposit where zeolite content varied in the range of 75–80%, by hydrothermal treatment. Hydrothermal synthesis was carried out in the temperature range from 90 to 300°С, the concentration range of the KOH + NaOH thermal solution from 10 to 35% and KCl + NaCl mineralizer of 3–20%, reaction time of 10–100 hours. Areas of existence of chabazite and faujasite with a 100% degree of crystallinity and phase purity were established: KOH + NaOH concentration of 15–20%, KCl + NaCl concentration of 10–15%, temperature at 230°С, processing time of 100 hours, and KOH + NaOH concentration of 20–30%, concentration of KCl + NaCl of 5–10%, temperature at 250°С, processing time of 50 hours, respectively. The formation of these zeolites was confirmed by X-ray phase analysis. The influence of temperature, the concentration of thermal solution and mineralizer, time of synthesis on the crystallization process was studied, and it was shown that a change in the above regions of existence of these zeolites contributes to the production of these zeolites in association with other zeolites and hydrosodalite.





Similar content being viewed by others
REFERENCES
Izidoro, J.C. and Fungaro, D.A., Dos Santos, F.S., Wang, S., Characteristics of Brazilian coal fly ashes and their synthesized zeolites, Fuel Process. Technol., 2012, vol. 97, p. 38.
Zhang, M., Zhang, H., Xua, D., Hanb, L., Niuc, D., Tiand, B., Zhang, J., Zhang, L., and Wua, W., Removal of ammonium from aqueous solutions using zeolite synthesized from fly ash by a fusion method, Desalination, 2011, vol. 271, nos. 1–3, p. 111.
Reinoso, D., Adrover, M., and Pedernera, M., Green synthesis of nanocrystalline faujasite zeolite, Ultrason. Sonochem., 2018, vol. 42, p. 303.
Kunecki, P., Panek, R., Wdowin, M., and Franus, W., Synthesis of faujasite (FAU) and tschernichite (LTA) type zeolites as a potential direction of the development of lime Class C fly ash, Int. J. Miner. Process., 2017, vol. 166, p. 69.
Belviso, C., Cavalcante, F., Huertas, F.J., Lettino, A., Ragone, P., and Fiore, S., The crystallisation of zeolite (X- and A-type) from fly ash at 25°C in artificial sea water, Micropor. Mesopor. Mater., 2012, vol. 162, p. 115.
Bo, W. and Hongzhu, M., Factors affecting the synthesis of microsized NaY zeolite, Micropor. Mesopor. Mater., 1998, vol. 25, nos. 1–3, p. 131.
Martin, N., Moliner, M., and Corma, A., High yield synthesis of high-silica chabazite by combining the role of zeolite precursors and tetraethylammonium: SCR of NOx , Chem. Commun., 2015, vol. 51, no. 49, p. 9965.
Liu, B., Zheng, Y., Hu, N., Gui, T., Li, Y., Zhang, F., Zhou, R., Chen, X., and Kita, H., Synthesis of low-silica CHA zeolite chabazite in fluoride media without organic structural directing agents and zeolites, Micropor. Mesopor. Mater., 2014, vol. 196, p. 270.
Bostrom, Z., Arstad, B., and Lillerud, K., Preparation of high silica chabazite with controllable particle size, Micropor. Mesopor. Mater., 2014, vol. 195, p. 294.
Dang, L.V., Le, S.T., Lobo, R.F., Pham, T.D., Hydrothermal synthesis of alkali-free chabazite zeolites, J. Porous Mater., 2020, vol. 27, no. 3, p. 1481.
Sanhueza Núñez, V. and Bennun Torres, L., Synthesis of zeolitic materials from volcanic ash in presence and absence of cetyltrimethylammonium bromide, Rev. Int. Contam. Ambiental, 2015, vol. 31, no. 2, p. 185.
Guo, Y., Sun, T., Gu, Y., Liu, X., Ke, Q., Wei, X., and Wang, S., Rational synthesis of chabazite (CHA) zeolites with controlled Si/Al ratio and their CO2/CH4/N2 adsorptive separation performances, Chem. Asian J., 2018, vol. 13, no. 21, p. 3222.
Che, S., Du, T., Zhu, S., Fang, X., and Wang, Y., Eco-friendly synthesis of kaolin-based chabazite for CO2 capture, J. Ceram. Soc. Jpn., 2019, vol. 127, no. 9, p. 606.
Hiromichi, A., Yuta, T., Yoshiteru, I., and Erni, J., Synthesis of chabazite and merlinoite for Cs+ adsorption and immobilization properties by heat-treatment, Solid State Sci., 2020, vol. 100, p. 106.
Shirazi, S. and Ashrafizadeh, S.N., Dehydration of natural gas using synthesized chabazite zeolite membranes, IAChE J., 2015, vol. 12, no. 1, p. 13.
Nasser, G.A., Muraza, O., Nishitoba, T., Malaibari, Z., Yamani, Z.H., Al-Shammari, T.K., and Yokoi, T., Microwave-assisted hydrothermal synthesis of CHA zeolite for methanol-to-olefins reaction, Ind. Eng. Chem. Res., 2019, vol. 58, no. 1, p. 60.
Motazedi, K., Mahinpey, N., and Karami, D., Preparation and application of faujasite-type Y zeolite-based catalysts for coal pyrolysis using sodium silicate solution and colloidal silica as silicon source, Chem. Eng. Commun., 2016, vol. 203, no. 3, p. 300.
Arryanto, S. and Arryanto, Y., Synthesis of faujasite from fly ash and its applications for hydrocracking of petroleum distillates, Bul. Chem. R. Eng. Cat., 2007, vol. 2, nos. 2–3, p. 45.
Ilao, M.C., Yamamoto, H., and Segawa, K., Shape-selective methylamine synthesis over small-pore zeolite catalysts, J. Catal., 1996, vol. 161, no. 1, p. 20.
Moneim, M. and Ahmed, E., Synthesis of faujasite from Egyptian clays: Characterizations and removal of heavy metals, Geomaterials, 2015, vol. 5, no. 02, p. 68.
Sang, S., Liu, Z., Tian, P., Liu, Z., Qu, L., and Zhang, Y., Synthesis of small crystals zeolite NaY, Mater. Lett., 2006, vol. 60, nos. 9–10, p. 1131.
Chaves, T., Pastore, H., and Cardoso, D., A simple synthesis procedure to prepare nanosized faujasite crystals, Micropor. Mesopor. Mater., 2012, vol. 161, p. 67.
Huang, Y., Wang, K., Dong, D., Li, D., Hill, M., Hill, A., and Wang, H., Synthesis of hierarchical porous zeolite NaY particles with controllable particle sizes, Micropor. Mesopor. Mater., 2010, vol. 127, no. 3, p. 167.
Kim, Y., Jeon, J., Hwang, J., Kim, S., and Kim, W., Influencing factors on rapid crystallization of high silica nano-sized zeolite Y without organic template under atmospheric pressure, J. Porous Mater., 2009, vol. 16, p. 299.
Nouri, A., Jafari, M., Kazeminoghadam, M., and Mohammadi, T., Effects of hydrothermal parameters on the synthesis of nanocrystalline zeolite NaY, Clays Clay Miner., 2012, vol. 60, no. 6, p. 610.
Zhao, Y., Liu, Z., Li, W., Zhao, Y., Pan, H., Liu, Y., Li, M., Kong, L., and He, M., Synthesis, characterization, and catalytic performance of high-silica Y zeolites with different crystallite size, Micropor. Mesopor. Mater., 2013, vol. 167, p. 102.
Valtchev, V. and Bozhilov, K., Transmission electron microscopy study of the formation of FAU-Type zeolite at room temperature, J. Phys. Chem. B, 2004, vol. 108, no. 40, p. 15587.
Bernamas, R., Bengueddach, A., and Di Renzo, F., Effectiveness of the tetramethylammonium size-modifier in the synthesis of faujasite nanocrystals, Catal. Today, 2014, vol. 227, p. 33.
Ayoola, A.A., Hymore, F.K., Omodara, J.O., Oyeniyi, A.E., Ojo, S.F., and Chisom, U.C., Effect of crystallization time on the synthesis of zeolite Y from Elefun kaolinite clay, Int. J. Appl. Eng. Res., 2017, vol. 12, no. 21, p. 10981.
Calligaris, M., Nardin, G., and Randaccio, L., Cation site location in hydrated chabazites: Crystal structure of potassium- and silver-exchanged chabazites, Zeolites, 1983, vol. 3, no. 3, p. 205.
Baur, W.H., On the cation and water positions in faujasite, Am. Mineral., 1964, vol. 49, nos. 5–6, p. 697.
Treacy, M. and Higgins, J., Collection of Simulated XRD Powder Patterns for Zeolites, New York: Elsevier, 2001.
Fan, Q., A new method of calculating interplanar spacing: the position-factor method, J. Appl. Crystallogr., 2012, vol. 45, no. 6, p. 1303.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Mamedova, G.A. Effect of Synthesis Conditions on the Crystallization Process of Faujasite and Chabazite on the basis of Nakhchivan Zeolitic Tuff. Theor Found Chem Eng 56, 783–790 (2022). https://doi.org/10.1134/S0040579522050281
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579522050281