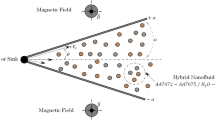
Abstract
The effect of the hydrodynamic situation (mainly conditions of mixing of the solutions of the reagents) on the composition and sizes of the nanoparticles being formed in an impinging-jet microreactor (IJMR) upon the impinging of the jets of the reagents—aqueous solutions of lanthanum nitrate and ammonium dihydrogen phosphate—is studied. Unique conditions are generated in the IJMR providing a short-term contact of the jets of the solutions that move at a high velocity (of about 10–20 m/s). The characteristics of turbulence in the IJMR are calculated. It is shown that the rate of dissipation of turbulent kinetic energy in the zone of impinging of the jets may reach 107–109 W/kg, which is comparable to the level of dissipation of energy in ultrasonic baths and is several orders of magnitude higher when compared to almost any other type of reactor. The effect of the region that has a size of the minimum Kolmogorov turbulence scale, i.e., a self-organizing “nanoreactor,” on the size of the particles being formed upon the deposition of particles with a complex composition is determined. Comparing the results of the calculation with the experimental data shows that, in some cases, the volume of the nanoreactor, taking into account the concentration of the solutions, determines the weight and size of the particles being formed. An explanation for the effect of a different influence of the velocity of impingement of the jets on the size of the nanoparticles being formed during the microreactor mixing of the reagents, depending on the characteristic features of the mechanisms of chemical reactions under the conditions of “soft” chemistry, is provided.








Similar content being viewed by others
REFERENCES
Hessel, V., Löwe, H., Müller, A., and Kolb, G., Chemical Micro Process Engineering: Processing and Plants, Weinheim: Wiley-VCH, 2005.
Capretto, L., Cheng, W., Hill, M., and Zhang, X., Micromixing within microfluidic devices, Top. Curr. Chem., 2011, vol. 304, p. 27. https://doi.org/10.1007/128_2011_150
Su, Y., Song, Y., and Xiang, L., Continuous-flow microreactors for polymer synthesis: Engineering principles and applications, Top. Curr. Chem., 2018, vol. 376, p. 44. https://doi.org/10.1007/s41061-018-0224-1
Popova, E.A., Abiev, R.Sh., Lappalainen, L.A., Svetlov, S.D., Andreeva, T.V., Trifonov, R.E., and Ostrovskii, V.A., Synthesis of 5-phenyltetrazole and its N-methyl derivatives in a microreactor, Chem. Biochem. Eng. Q., 2014, vol. 28, no. 2, p. 241.
Abiev R.Sh., Pavlyukova, Y.N., Nesterova, O.M., Svetlov, S.D., and Ostrovskii, V.A., Mass transfer intensification of 2-methyl-5-nitrotetrazole synthesis in two-phase liquid–liquid Taylor flow in microreactor, Chem. Eng. Res. Des., 2019, vol. 144, pp. 444–458. https://doi.org/10.1016/j.cherd.2019.01.033
Wörner, M., A correlation for the characteristic velocity ratio to predict hydrodynamics of capillary gas–liquid Taylor flow, Theor. Found. Chem. Eng., 2020, vol. 54, no. 1, pp. 3–16. https://doi.org/10.1134/S0040579520010236
Mei, M., Felis, F., Hébrard, G., Dietrich, N., and Loubière, K., Hydrodynamics of gas–liquid slug flows in a long in-plane spiral shaped milli-reactor, Theor. Found. Chem. Eng., 2020, vol. 54, no. 1, pp. 25–47. https://doi.org/10.1134/S0040579520010169
Abiev, R.Sh., Miniaturization as one of the paths to process intensification in chemical engineering, Theor. Found. Chem. Eng., 2020, vol. 54, no. 1, pp. 1–2. https://doi.org/10.1134/S0040579520300016
Haase, S., Bauer, T., Hilpmann, G., Lange, M., Ayubi, M.-M., and Abiev, R., Simultaneous detection of hydrodynamics, mass transfer and reaction rates in a three-phase microreactor, Theor. Found. Chem. Eng., 2020, vol. 54, no. 1, pp. 48–63. https://doi.org/10.1134/S0040579520010091
Kumar, R., Yadav, V., and Abiev, R.Sh., Concurrent removal of heat transfer and mass flow rate nonuniformities in parallel channels of microchannel heat sink, Theor. Found. Chem. Eng., 2020, vol. 54, no. 1, pp. 77–90. https://doi.org/10.1134/S004057952001011X
Meskin, P.E., Gavrilov, A.I., Maksimov, V.D., Ivanov, V.K., and Churagulov, B.P., Hydrothermal/microwave and hydrothermal/ultrasonic synthesis of nanocrystalline titania, zirconia, and hafnia, Russ. J. Inorg. Chem., 2007, vol. 52, no. 11, p. 1648. https://doi.org/10.1134/s0036023607110022
Proskurina, O.V., Tomkovich, M.V., Bachina, A.K., Sokolov, V.V., Danilovich, D.P., Panchuk, V.V., Semenov, V.G., and Gusarov, V.V., Formation of nanocrystalline BiFeO3 under hydrothermal conditions, Russ. J. Gen. Chem., 2017, vol. 87, no. 11, pp. 2507–2515. https://doi.org/10.1134/S1070363217110019
Chen, C., Cheng, J., Yu, S., Che, L., and Meng, Z., Hydrothermal synthesis of perovskite bismuth ferrite crystallites, J. Cryst. Growth, 2006, vol. 291, p. 135.
Zhu, Z., Flash nanoprecipitation: Prediction and enhancement of particle stability via drug structure, Mol. Pharm., 2014, vol. 11, p. 776.
Margulis, K., Magdassi, S., Lee, H.S., and Macosko, C.W., Formation of curcumin nanoparticles by flash nanoprecipitation from emulsions, J. Colloid Interface Sci., 2014, vol. 434, p. 65.
Han, J., Zhu, Z., Qian, H., Wohl, A.R., Beaman, C.J., Hoye, T.R., and Macosko, C.W., A simple confined impingement jets mixer for flash nanoprecipitation, J. Pharm. Sci., 2012, vol. 101, no. 10, p. 4018.
Ravi Kumar, D.V., Prasad, B.L.V., and Kulkarni, A.A., Impinging jet micromixer for flow synthesis of nanocrystalline MgO: Role of mixing/impingement zone, Ind. Eng. Chem. Res., 2013, vol. 52, p. 17376. https://doi.org/10.1021/ie402012x
Abiev, R.S., Almyasheva, O.V., Izotova, S.G., and Gusarov, V.V., Synthesis of cobalt ferrite nanoparticles by means of confined impinging-jets reactors, J. Chem. Technol. Appl., 2017, vol. 1, no. 1, pp. 7–13. https://doi.org/10.35841/chemical-technology.1.1.7-13
Kawase, M. and Miura, K., Fine particle synthesis by continuous precipitation using a tubular reactor, Adv. Powder Technol., 2007, vol. 18, no. 6, p. 725.
Che, D., Zhu, X., Liu, P., Duan, Y., Wang, H., Zhang, Q., and Li, Y., A facile aqueous strategy for the synthesis of high-brightness LaPO4:Eu nanocrystals via controlling the nucleation and growth process, J. Lumin., 2014, vol. 153, pp. 369–374. https://doi.org/10.1016/j.jlumin.2014.03.028
Nightingale, A.M. and deMello, J.C., Segmented flow reactors for nanocrystal synthesis, Adv. Mater., 2013, vol. 25, p. 1813.
Nightingale, A.M., Krishnadasan, S.H., Berhanu, D., Niu, X., Drury, C., McIntyre, R., Valsami-Jones, E., and deMello, J.C., A stable droplet reactor for high temperature nanocrystal synthesis, Lab Chip, 2011, vol. 11, p. 1221.
Doh, I., Erdem, E.Y., and Pisano, A.P., Trapping and collection of uniform size droplets for nanoparticle synthesis, Appl. Phys. Lett., 2012, vol. 100, p. 074106.
Salvador, H.M.M., Fully resolved dynamics of mixing in confined impinging jets reactors, Master Dissertation in Computational Mechanics, Porto: Univ. do Porto, 2015.
Proskurina, O.V., Nogovitsin, I.V., Il’ina, T.S., Danilovich, D.P., Abiev, R.Sh., and Gusarov, V.V., Formation of BiFeO3 nanoparticles using impinging jets microreactor, Russ. J. Gen. Chem., 2018, vol. 88, no. 10, pp. 2139–2143. https://doi.org/10.1134/S1070363218100183
Proskurina, O.V., Abiev, R.S., Danilovich, D.P., Panchuk, V.V., Semenov, V.G., Nevedomsky, V.N., and Gusarov, V.V., Formation of nanocrystalline BiFeO3 during heat treatment of hydroxides co-precipitated in an impinging-jets microreactor, Chem. Eng. Process., 2019, vol. 143, p. 107598. https://doi.org/10.1016/j.cep.2019.107598
Proskurina, O.V., Sivtsov, E.V., Enikeeva, M.O., Sirotkin, A.A., Abiev, R.Sh., and Gusarov, V.V., Formation of rhabdophane-structured lanthanum orthophosphate nanoparticles in an impinging-jets microreactor and rheological properties of sols based on them, Nanosyst.: Phys., Chem., Math., 2019, vol. 10, no. 2, p. 206. https://doi.org/10.17586/2220-8054-2019-10-2-206-214
Hasson, D. and Peck, R.E., Thickness distribution in a sheet formed by impinging jets, AIChE J., 1964, vol. 10, no. 5, p. 752.
Ibrahim, E. and Przekwas, A., Impinging jets atomization, Phys. Fluids A, 1991, vol. 3, p. 2981.
Ashgriz, N., Impinging jet atomization, Handbook of Atomization and Sprays: Theory and Applications, Ashgriz, N., Ed., Boston: Springer, 2011, ch. 30, pp. 685–707. https://doi.org/10.1007/978-1-4419-7264-4_30
Li, R. and Ashgriz, N., Characteristics of liquid sheets formed by two impinging jets, Phys. Fluids, 2006, vol. 18, p. 087104.
Chen, X., Ma, D., Yang, V., and Popinet, S., High-fidelity simulations of impinging jet atomization, Atomization Sprays, 2013, vol. 23, no. 14, p. 1079.
Faber, T.E., Fluid Dynamics for Physicists, Cambridge: Cambridge Univ. Press, 1997.
Choo, Y.-J. and Kang, B.-S., The velocity distribution of the liquid sheet formed by two low-speed impinging jets, Phys. Fluids, 2002, vol. 14, no. 2, p. 622. https://doi.org/10.1063/1.1429250
Ashgriz, N., Brocklehurst, W., and Talley, D., Mixing mechanisms in a pair of impinging jets, J. Propul. Power, 2001, vol. 17, no. 3, pp. 736–749. https://doi.org/10.2514/2.5803
Abiev, R.Sh., Al’myasheva, O.V., Gusarov, V.V., and Izotova, S.G., RF Patent 2625981, Izobret., Polezn. Modeli, 2017, no. 20.
Perry’s Chemical Engineers’ Handbook, Green, D.W. and Perry, R.H., Eds., New York: McGraw-Hill, 2007.
Bałdyga, J. and Bourne, J.R., Turbulent Mixing and Chemical Reactions, Chichester: Wiley, 1999.
Atiemo-Obeng, V.A. and Calabrese, R.V., Rotor–stator mixing devices, Handbook of Industrial Mixing: Science and Practice, Paul, E.L., Atiemo-Obeng, V.A., and Kresta, S.M., Eds., Hoboken, N.J.: Wiley, 2004, ch. 8, pp. 479–505. https://doi.org/10.1002/0471451452.ch8
Davies, J.T., A physical interpretation of drop sizes in homogenizers and agitated tanks, including the dispersion of viscous oils, Chem. Eng. Sci., 1987, vol. 42, p. 1671.
Guichardon, P. and Falk, L., Characterisation of micromixing efficiency by the iodide-iodate reaction system. Part I: Experimental procedure, Chem. Eng. Sci., 2000, vol. 55, p. 4233. https://doi.org/10.1016/S0009-2509(00)00068-3
Commenge, J.-M. and Falk, L., Villermaux–Dushman protocol for experimental characterization of micromixers, Chem. Eng. Process., 2011, vol. 50, p. 979.
Jasińska, M., Test reactions to study efficiency of mixing, Chem. Process Eng., 2015, vol. 36, no. 2, p. 171.
Qadeer, R. and Khalid, N., Influence of concentration and temperature on viscosity of nitrate solutions of some trivalent lanthanides, J. Chem. Eng. Data, 2004, vol. 49, p. 892.
Almjasheva, O.V., Lomanova, N.A., Popkov, V.I., Proskurina, O.V., Tugova, E.A., and Gusarov, V.V., The minimal size of oxide nanocrystals: Phenomenological thermodynamic vs crystal-chemical approaches, Nanosyst.: Phys., Chem., Math., 2019, vol. 10, no. 4, p. 428. https://doi.org/10.17586/2220-8054-2019-10-4-428-437
Almjasheva, O.V. and Gusarov, V.V., Metastable clusters and aggregative nucleation mechanism, Nanosyst.: Phys., Chem., Math., 2014, vol. 5, no. 3, pp. 405–416.
Almjasheva, O.V., Formation and structural transformations of nanoparticles in the TiO2–H2O system, Nanosyst.: Phys., Chem., Math., 2016, vol. 7, no. 6, pp. 1031–1049. https://doi.org/10.17586/2220-8054-2016-7-6-1031-1049
Ivanov, V.K., Fedorov, P.P., Baranchikov, A.Ye., and Osiko, V.V., Oriented attachment of particles: 100 years of investigations of non-classical crystal growth, Russ. Chem. Rev., 2014, vol. 83, no. 12, p. 1204. https://doi.org/10.1070/RCR4453
Funding
This work was financially supported by the Russian Foundation for Basic Research (project no. 18-29-12119).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Boltukhina
Rights and permissions
About this article
Cite this article
Abiev, R.S., Proskurina, O.V., Enikeeva, M.O. et al. Effect of Hydrodynamic Conditions in an Impinging-Jet Microreactor on the Formation of Nanoparticles Based on Complex Oxides. Theor Found Chem Eng 55, 12–29 (2021). https://doi.org/10.1134/S0040579521010012
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579521010012