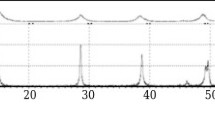
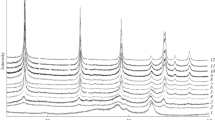
Abstract
A method is proposed for the synthesis of nanoscale boehmite powder at low temperatures of hydrothermal treatment in 1.5 wt % HCl solution at 150 and 170°C and in water at 80, 100, and 130°C. The optimal parameters of hydrothermal treatment are found. The conversion stages of γ-Al2O3 to boehmite (AlOOH) are found; the process is solid-phase (topochemical). The physical and technological properties of boehmite nanopowder are studied. We have found that boehmite possesses similar properties despite the temperature of hydrothermal treatment.







Similar content being viewed by others
REFERENCES
Oh, M.-H., Nho, J.-S., Cho, S.-B., et al., Novel method to control the size of well-crystalline ceria particles by hydrothermal method, Mater. Chem. Phys., 2010, vol. 124, no. 1, p. 134. https://doi.org/10.1016/j.matchemphys.2010.06.004
Quan, Y., Fang, D., Zhang, X., et al., Synthesis and characterization of gallium oxide nanowires via a hydrothermal method, Mater. Chem. Phys., 2010, vol. 121, nos. 1–2, p. 142. https://doi.org/10.1016/j.matchemphys.2010.01.009
Shan, H.-Y., Li, J., Li, S., et al., Epitaxial ZnO films grown on ZnO-buffered c-plane sapphire substrates by hydrothermal method, Appl. Surf. Sci., 2010, vol. 256, no. 22, p. 6743. https://doi.org/10.1016/j.apsusc.2010.04.083
Kim, S.-J., Kim, H.-H., Kwon, J.-B., et al., Novel fabrication of various size ZnO nanorods using hydrothermal method, Microelectron. Eng., 2010, vol. 87, nos. 5–8, p. 1534. https://doi.org/10.1016/j.mee.2009.11.033
Chen, Z. and He, X., Low-temperature preparation of nanoplated bismuth titanate microspheres by a sol-gel-hydrothermal method, J. Alloys Compd., 2010, vol. 497, nos. 1–2, p. 312. https://doi.org/10.1016/j.jallcom.2010.03.053
Ding, M., Zhao, D., Yao, B., et al., The p-type ZnO film realized by a hydrothermal treatment method, Appl. Phys. Lett., 2011, vol. 98, no. 6, article no. 062102. https://doi.org/10.1063/1.3549304
Sreekantan, S. and Wei, L.C., Study on the formation and photocatalytic activity of titanate nanotubes synthesized via hydrothermal method, J. Alloys Compd., 2010, vol. 490, nos. 1–2, p. 436. https://doi.org/10.1016/j.jallcom.2009.10.030
Liu, R., Yang, W.D., and Chueng, H.J., Preparation and visible-light photocatalytic activity of TiO2 nanotubes from a hydrothermal method, Adv. Mater. Res., 2011, vols. 197–198, p. 786. https://doi.org/10.4028/www.scientific.net/amr.197-198.786
Kometani, N. and Teranishi, T., Preparation of size-controlled silver nanoparticles by the hydrothermal method, Phys. Status Solidi C., 2010, vol. 7, nos. 11–12, p. 2644. https://doi.org/10.1002/pssc.200983783
Manikandan, V., Jayanthi, P., Priyadharsan, A., et al., Green synthesis of pH-responsive Al2O3 nanoparticles: Application to rapid removal of nitrate ions with enhanced antibacterial activity, J. Photochem. Photobiol., A, 2019, vol. 371, p. 205. https://doi.org/10.1016/j.jphotochem.2018.11.009
Ribut, S.H., Abdullah, C.A.C., Mustafa, M., et al., Influence of pH variations on zinc oxide nanoparticles and their antibacterial activity, Mater. Res. Express, 2019, vol. 6, no. 2, article no. 025016. https://doi.org/10.1088/2053-1591/aaecbc
Mohanraj, V., Jayaprakash, R., Chandrasekaran, J., et al., Influence of pH on particle size, band-gap and activation energy of CdS nanoparticles synthesized at constant frequency ultrasonic wave irradiation, Mater. Sci. Semicond. Process., 2017, vol. 66, p. 131. https://doi.org/10.1016/j.mssp.2017.04.006
Tang, Z., Kwon, H., Yi, M.Y., et al., Role of halide ions for controlling morphology of copper nanocrystals in aqueous solution, ChemistrySelect, 2017, vol. 2, no. 17, p. 4655. https://doi.org/10.1002/slct.201701173
Wan, Y.Y. and Zhou, X.P., Formation mechanism of hafnium oxide nanoparticles by a hydrothermal route, RSC Adv., 2017, vol. 7, no. 13, p. 7763. https://doi.org/10.1039/c6ra26663k
Mestanza, S.N.M., Ribeiro, A.O., De Souza Ribeiro, C.S., et al., Study of the influence of dynamics variables on the growth of silica nanoparticles, Inorg. Nano-Met.Chem., 2017, vol. 47, no. 6, p. 824. https://doi.org/10.1080/15533174.2016.1212226
Podlogar, M., Recnik, A., Yilmazoglu, G., et al., The role of hydrothermal pathways in the evolution of the morphology of ZnO crystals, Ceram. Int., 2016, vol. 42, no. 14, p. 15358. https://doi.org/10.1016/j.ceramint.2016.06.181
Egorova, S.R., Bekmukhamedov, G.E., Mukhamed’yarova, A.N., et al., On the nature of phase conversions and transformations in porous system in hydrothermal processing of χ-Al2O3 into boehmite, Russ. J. Appl. Chem., 2016, vol. 89, no. 5, p. 703. https://doi.org/10.1134/S1070427216050049
Akiba, H., Ichiji, M., Nagao, H., et al., Effect of seeding and pH conditions on the size and shape of Au nanoparticles in reduction crystallization, Chem. Eng. Technol., 2015, vol. 38, no. 6, p. 1068. https://doi.org/10.1002/ceat.201400671
Hai, C.X., Zhang, L.J., Zhou, Y., et al., Phase transformation and morphology evolution characteristics of hydrothermally prepared boehmite particles, J. Inorg. Organomet. Polym. Mater., 2018, vol. 28, no. 3, p. 643. https://doi.org/10.1007/s10904-017-0756-9
Padilla, I., Lopez-Andres, S., and Lopez-Delgado, A., Effects of Different Raw Materials in the Synthesis of Boehmite and γ- and α-Alumina, J. Chem., 2019, article no. 5353490. https://doi.org/10.1155/2016/5353490
Panasyuk, G.P., Kozerozhets, I.V., Semenov, E.A., et al., Mechanism of phase transformations of γ-Al2O3 and Al(OH)3 into boehmite (AlOOH) during hydrothermal treatment, Inorg. Mater., 2019, vol. 55, no. 9, p. 929. https://doi.org/10.1134/S0020168519090139
Panasyuk, G.P., Belan, V.N., Voroshilov, I.L., et al., The study of hydrargillite and γ-alumina conversion process in boehmite in different hydrothermal media, Theor. Found. Chem. Eng., 2013, vol. 47, no. 4, p. 415. https://doi.org/10.1134/S0040579513040143
Panasyuk, G.P., Belan, V.N., Voroshilov, I.L., et al., Hydrargillite → boehmite transformation, Inorg. Mater., 2010, vol. 46, no. 7, p. 747. https://doi.org/10.1134/S0020168510070113
Panasyuk, G.P., Semenov, E.A., Kozerozhets, I.V., Azarova, L.A., Voroshilov, I.L., Belan, V.N., and Pershikov, S.A., RF Patent 2625388, 2015.
Panasyuk, G.P., Kozerozhets, I.V., Danchevskaya, M.N., et al., A new method for synthesis of fine crystalline magnesium aluminate spinel, Dokl. Chem., 2019, vol. 487, no. 2, p. 218. https://doi.org/10.1134/S0012500819080019
Panasyuk, G.P., Azarova, L.A., Belan, V.N., et al., Preparation of fine-grained corundum powders with given properties: Crystal size and habit control, Theor. Found. Chem. Eng., 2018, vol. 52, no. 5, p. 879. https://doi.org/10.1134/S0040579518050202
Panasyuk, G.P., Semenov, E.A., Kozerozhets, I.V., et al., Production of high-flexural-strength corundum ceramics, Dokl. Chem., 2019, vol. 485, no. 2, p. 116. https://doi.org/10.1134/S0012500819040049
Panasyuk, G.P., Semenov, E.A., Kozerozhets, I.V., et al., A new method of synthesis of nanosized boehmite (AlOOH) powders with a low impurity content, Dokl. Chem., 2018, vol. 483, p. 272. https://doi.org/10.1134/S0012500818110022
Chen, Y.G., Huo, W.L., Zhang, X.Y., et al., Ultrahigh-strength alumina ceramic foams via gelation of foamed boehmite sol, J. Am. Ceram. Soc., 2019, vol. 102, no. 9, p. 5503. https://doi.org/10.1111/jace.16378
Kamari, M., Shafiee, S., Salimi, F., et al., Comparison of modified boehmite nanoplatelets and nanowires for dye removal from aqueous solution, Desalin. Water Treat., 2019, vol. 161, p. 304. https://doi.org/10.5004/dwt.2019.24295
Rajamani, M. and Rajendrakumar, K., Chitosan-boehmite desiccant composite as a promising adsorbent towards heavy metal removal, J. Environ. Manage., 2019, vol. 244, p. 257. https://doi.org/10.1016/j.jenvman.2019.05.056
Dubey, S.P., Dwivedi, A.D., Sillanpaa, M., et al., Adsorption of As(V) by boehmite and alumina of different morphologies prepared under hydrothermal conditions, Chemosphere, 2017, vol. 169, p. 99. https://doi.org/10.1016/j.chemosphere.2016.11.052
Panasyuk, G.P., Kozerozhets, I.V., Semenov, E.A., et al., A new method for producing a nanosized γ-Al2O3 powder, Russ. J. Inorg. Chem., 2018, vol. 63, no. 10, p. 1303. https://doi.org/10.1134/S0036023618100157
Panasyuk, G.P., Kozerozhets, I.V., Voroshilov, I.L., et al., The thermodynamic properties and role of water contained in dispersed oxides in precursor-boehmite conversion, based on the example of aluminum hydroxide and oxide under hydrothermal conditions in different environments, Russ. J. Phys. Chem. A, 2015, vol. 89, no. 4, p. 592. https://doi.org/10.1134/S0036024415040196
Funding
This work was performed as part of the state assignment of the Kurnakov Institute of General and Inorganic Chemistry (Russian Academy of Science) in the field of fundamental scientific studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Tulyabaev
Rights and permissions
About this article
Cite this article
Kozerozhets, I.V., Panasyuk, G.P., Semenov, E.A. et al. Synthesis of Boehmite Nanosized Powder (AlOOH) at Low Temperatures of Hydrothermal Treatment. Theor Found Chem Eng 54, 465–473 (2020). https://doi.org/10.1134/S0040579520030082
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579520030082