Abstract
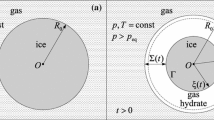
Based on the diffusion theory, a gas–solid reaction model for gas hydrate formation from monosize ice powders was constructed and an effective diffusion coefficient through the gas hydrate shell was obtained by solving the diffusion equation. This model can be applied for a gas–solid reaction process with gas pressure drop during hydrate formation. Two cases termed as M1 and M2 were discussed and compared with each other. In the M1 case, the spherical particle geometry was assumed to be squeezed by adjacent particles. In the M2 case, the effect of adjacent particles on the object particle geometry was neglected. The results showed that the gas effective diffusion coefficient was a critical parameter for accurately simulating the reaction process, and it was found to vary during hydrate formation. In this model, the time-dependent gas effective diffusion coefficient was calculated by means of the measured gas pressure during hydrate formation, and the degrees of hydrate formation were obtained. In addition, the geometrical changes of the hydrate shell and ice powders were obtained during hydrate formation.








Similar content being viewed by others
REFERENCES
Ribeiro, C.P. and Lage, P.L.C., Modelling of hydrate formation kinetics: State-of-the-art and future directions, Chem. Eng. Sci., 2008, vol. 63, no. 8, p. 2007.
Gudmundsson, J.S., Andersson, V., Levik, O.I., and Mork, M., Hydrate technology for capturing stranded gas, Gas Hydrates: Challenges for the Future, Holder, G.D. and Bishnoi, P.R., Eds., Annals of the New York Academy of Sciences, vol. 912, New York: New York Academy of Sciences, 2000, p. 403.
Vorotyntsev, V.M., Malyshev, V.M., Taraburov, P.G., and Mochalov, G.M., Separation of gas mixture by the continuous gas hydrate crystallization, Theor. Found. Chem. Eng., 2001, vol. 35, no. 6, p. 513.
Vorotyntsev, V.M. and Malyshev, V.M., Gas hydrates: Nanosized phases in the separation and purification of substances by crystallization, Russ. Chem. Rev., 2011, vol. 80, no. 10, p. 971.
Sloan, E.D., Clathrate Hydrates of Natural Gases, New York: Marcel Dekker, 1998, 2nd ed.
Takeya, S., Hondoh, T., and Uchida, T., In situ observation of CO2 hydrate by X-ray diffraction, Gas Hydrates: Challenges for the Future, Holder, G.D. and Bishnoi, P.R., Eds., Annals of the New York Academy of Sciences, vol. 912, New York: New York Academy of Sciences, 2000, p. 973.
Henning, R.W., Schultz, A.J., Thieu, V., and Halpern, Y., Neutron diffraction studies of CO2 clathrate hydrate: Formation from deuterated ice, J. Phys. Chem. A., 2000, vol. 104, no. 21, p. 5066.
Hwang, M.J., Wright, D.A., Kapur, A., and Holder, G.D., An experimental study of crystallization and crystal growth of methane hydrates from melting ice, J. Inclusion Phenom. Mol. Recognit. Chem., 1990, vol. 8, nos. 1–2, p. 103.
Kuhs, W.F., Klapproth, A., Gotthardt, F., Techmer, K., and Heinrichs, T., The formation of meso- and macroporous gas hydrates, Geophys. Res. Lett., 2000, vol. 27, no. 18, p. 2929.
Kuhs, W.F., Staykova, D.K., and Salamatin, A.N., Formation of methane hydrate from polydisperse ice powders, J. Phys. Chem. B., 2006, vol. 110, no. 26, p. 13283.
Klapproth, A., Goreshnik, E., Staykova, D., Klein, H., and Kuhs, W.F., Structural studies of gas hydrates, Can. J. Phys., 2003, vol. 81, nos. 1–2, p. 503.
Staykova, D.K., Hansen, T., Salamatin, A.N., and Kuhs, W.F., Kinetic diffraction experiments on the formation of porous gas hydrates, Proc. 4th Int. Conf. on Gas Hydrates, Yokohama, Japan, 2002, p. 537.
Staykova, D.K., Kuhs, W.F., Salamatin, A.N., and Hansen, T., Formation of porous gas hydrates from ice powders: Diffraction experiments and multistage model, J. Phys. Chem. B., 2003, vol. 107, no. 37, p. 10299.
Halpern, Y., Thieu, V., Henning, R.W., Wang, X.P., and Schultz, A.J., Time-resolved in situ neutron diffraction studies of gas hydrate: Transformation of structure II (sII) to structure I (sI), J. Am. Chem. Soc., 2001, vol. 123, no. 51, p. 12826.
Wang, X.P., Schultz, A.J., and Halpern, Y., Kinetics of methane hydrate formation from polycrystalline deuterated ice, J. Phys. Chem. A., 2002, vol. 106, no. 32, p. 7304.
Homma, S., Ogata, S., Koga, J., and Matsumoto, S., Gas-solid reaction model for a shrinking spherical particle with unreacted shrinking core, Chem. Eng. Sci., 2005, vol. 60, no. 18, p. 4971.
Salamatin, A.N. and Kuhs, W.F., Formation of porous gas hydrates, Proc. 4th Int. Conf. on Gas Hydrates, Yokohama, Japan, 2002, p. 766.
Arzt, E., The influence of an increasing particle coordination on the densification of spherical powders, Acta Metall., 1982, vol. 30, no. 10, p. 1883.
Crank, J., The Mathematics of Diffusion, Oxford: Clarendon, 1975, 2nd ed.
Vlasov, V.A., Phenomenological diffusion theory of formation of gas hydrate from ice powder, Theor. Found. Chem. Eng., 2012, vol. 46, no. 6, p. 576.
Lee, H., Seo, Y., Seo, Y.T., Moudrakovski, I.L., and Ripmeester, J.A., Recovering methane from solid methane hydrate with carbon dioxide, Angew. Chem., Int. Ed., 2003, vol. 42, no. 41, p. 5048.
Vlasov, V.A., Formation and dissociation of gas hydrate in terms of chemical kinetics, React. Kinet., Mech. Catal., 2013, vol. 110, no. 1, p. 5.
ACKNOWLEDGMENTS
This study has been supported by the National Key R&D Program of China (grant nos. 2017YFC0307305 and 2016YFC0304001) and the National Natural Science Foundation of China (grant nos. 51676024 and 51876029).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
APPENDIX
APPENDIX
The rate of the forward reaction of gas hydrate formation can be represented as
In the state of the thermodynamic equilibrium of the ice–gas hydrate–gas system, the following condition is satisfied:
The rate of decrease in the amount of the gas on the surface is
For the diffused gas was fully involved in the generating reaction, the internal boundary condition is
As it has been assumed, the diffusion is pseudo-steady where it is not in the external boundary; obviously, the following equation is reasonable:
The solution of the above equation is \(c\left( {r,t} \right) = \frac{{a\left( t \right)}}{r} + b\left( t \right)\).
Due to the thermodynamic conditions of the external boundary are varied, we have
The improved solution is
For \(B\left( t \right) = 0,~\,\,\,{{R}_{{\text{i}}}}\left( t \right)~\, \leqslant r~ < ~{{R}_{{\text{h}}}}\left( t \right)\), we will obtain a unified solution.
In the external boundary condition,
In the internal boundary condition,
By (A.7) and (A.8), we will obtain \(a\left( t \right)\), \(b\left( t \right)\). At the external boundary of hydrate shell, B(t) is obtained by the real gas state equation. At internal boundary of the hydrate shell, while the discrete time is sufficiently small [17], the process is regarded as pseudo-steady state, and B(t) is equal to 0. Then, we obtain \({{\left. {B\left( t \right)} \right|}_{{r = {{R}_{{\text{h}}}}\left( t \right)}}} = \frac{1}{{6{{D}_{{{\text{eff}}}}}}}\frac{{\partial \left( {\frac{p}{{ZRT}}} \right)}}{{\partial t}},\) when \(p,T = {\text{const}},\)
The molar flux through the gas hydrate layer is
Because of molar conservation, we have the following formula:
According to (A.12), we have
Then, we obtain
The number of ice particles in the sample is as follows:
The variation in the molar flow rate of the gas around the individual hydrate particle is approximately equal to the molar flux through the hydrate layer:
According to molar conservation, we have
Combining formulas (A.13) and (A.15), we have formula (8). According to (A.18), we obtain formula (9).
NOTATION
C z | slope of the random density function |
c(r, t) | gas concentration in the layer of the gas hydrate, mol/m3 |
Δc | gas concentration variation, mol/m3 |
D eff | effective diffusion coefficient of the gas in the gas hydrate, m2/s |
k R | reaction rate constant for the formation of gas hydrate on the surface, m3n + 1/moln |
m i | total mass of ice, kg |
N | number of ice particles in the sample |
n | hydrate number |
p | gas pressure outside the particle, Pa |
p(t) | function of pressure over time, Pa |
p eq | pressure of the ice–gas hydrate–gas equilibrium, Pa |
Q | molar flux of the gas in the gas hydrate layer, mol/s |
R | gas constant, J/(mol s) |
R 0 | initial radius of the ice particle, m |
Rh(t) | outside radius of the gas hydrate layer, m |
Rh(t)* | outside radius of the gas hydrate layer, m |
Ri(t) | inside radius of the gas hydrate layer, m |
R form | rate of the reaction of gas hydrate formation on the surface, mol/s |
R dis | rate of the reaction of gas hydrate dissociation on the surface, mol/s |
r g | rate of decrease in the amount of gas on the surface during a chemical reaction, mol/s; mol/s |
T | temperature, K |
t | time, s |
Δt | discrete time, s |
V | volume, m3 |
Z | compressibility factor |
Z 0 | current coordination number |
Z eq | compressibility factor for a gas that is under equilibrium conditions |
δr | thickness of the hydrate shell at the origin time, m |
δt | time for hydrate shell formation, s |
η(t) | degree of hydrate formation |
ρi | density of ice, kg/m3 |
ρmi | molar density of ice, mol/m3 |
ρmh | molar density of the gas hydrate, mol/m3 |
Rights and permissions
About this article
Cite this article
Liu, W., Li, Y., Zhang, L. et al. Modeling Gas Hydrate Formation from Ice Powders Based on Diffusion Theory. Theor Found Chem Eng 53, 305–317 (2019). https://doi.org/10.1134/S0040579519020106
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579519020106