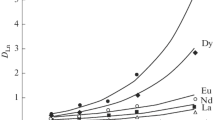
Abstract
The interphase distribution of lanthanide salts in ternary aqueous–organic systems of various compositions has been studied. It has been shown that the distribution of lanthanide salts in multicomponent aqueous–organic systems differs from their distribution in extraction systems. The order of extractability of lanthanide chlorides and lanthanide nitrates in a 1 : 1 : 1 system (0.5 M NaCl (NaNO3) in water–binary extractant in hexane–isopropyl alcohol) has been determined.



Similar content being viewed by others
REFERENCES
Michelsen, O.B. and Smutz, M., Separation of yttrium, holmium, and erbium with di-(2-ethylhexyl) phosphoric acid in chloride and nitrate systems, J. Inorg. Nucl. Chem., 1971, vol. 33, no. 1, pp. 265–278. https://doi.org/10.1016/0022-1902(71)80028-3
Minagawa, Y. and Yamaguchi, K., Relative extraction rates of rare earth ions from weakly acidic solution of binary mixture of rare earth chlorides by di-(2-ethyl-hexyl) phosphoric acid/kerosine, Hydrometallurgy, 1990, vol. 24, no. 3, pp. 333–350. https://doi.org/ 10.1016/0304-386X(90)90097-L
Kalyakin, S.N., Kuz’min, V.I., and Mulagaleeva, M.A., The application of binary extractants based on di-(2-ethyl-hexyl) phosphoric acid to the separation of lanthanides, Tsvetn. Met., 2011, no. 3, pp. 51–54.
Kalyakin, S.N., Kuz’min, V.I., and Mulagaleeva, M.A., Binary extraction of lanthanide(III) chlorides using carboxylates and dialkylphosphates of secondary and tertiary amines, Hydrometallurgy, 2015, vol. 151, pp. 116–121. https://doi.org/10.1016/j.hydromet.2014.11.013
Egorova, N.S., Belova, V.V., Voshkin, A.A., Zhilov, V.I., and Khol’kin, A.I., Extraction of lanthanide chlorides by binary extractants based on phosphinic acid derivatives, Russ. J. Inorg. Chem., 2005, vol. 50, no. 11, pp. 1781–1784.
Belova, V.V., Voshkin, A.A., Kholkin, A.I., and Payrtman, A.K., Solvent extraction of some lanthanides from chloride and nitrate solutions by binary extractants, Hydrometallurgy, 2009, vol. 97, nos. 3–4, pp. 198–203. https://doi.org/10.1016/j.hydromet.2009.03.004
Zakhodyaeva, Yu.A., Belova, V.V., Egorova, N.S., and Khol’kin, A.I., Extraction of rare earth metal salts using methyltrioctylammonium dialkyl phosphate and dialkyl phosphinate, Khim. Tekhnol., 2015, vol. 16, no. 1, pp. 23–29.
Kostanyan, A.E., Voshkin, A.A., and Kodin, N.V., Controlled-cycle pulsed liquid–liquid chromatography. A modified version of Craig’s counter-current distribution, J. Chromatogr. A, 2011, vol. 1218, no. 36, pp. 6135–6143. https://doi.org/10.1016/j.chroma.2010.12.103
Kostanyan, A.E., On influence of sample loading conditions on peak shape and separation efficiency in preparative isocratic counter-current chromatography, J. Chromatogr. A, 2012, vol. 1254, pp. 71–77. https:// doi.org/10.1016/j.chroma.2012.07.036
Kostanyan, A.E., On the description of liquid–liquid chromatography processes with a free stationary phase, Khim. Tekhnol., 2004, vol. 5, no. 8, pp. 39–42.
Kostanyan, A.E., Ignatova, S., Sutherland, I.A., Hewitson, P., Zakhodjaeva, Y.A., and Erastov, A.A., Steady-state and non-steady state operation of counter-current chromatography devices, J. Chromatogr. A, 2013, vol. 1314, pp. 94–105. https://doi.org/10.1016/ j.chroma.2013.08.100
Kostanyan, A.E., Erastov, A.A., and Shishilov, O.N., Multiple dual mode counter-current chromatography with variable duration of alternating phase elution steps, J. Chromatogr. A, 2014, vol. 1347, pp. 87–95. https:// doi.org/10.1016/j.chroma.2014.04.064
Kostanyan, A.E., Modeling of closed-loop recycling liquid–liquid chromatography: Analytical solutions and model analysis, J. Chromatogr. A, 2015, vol. 1406, pp. 156–164. https://doi.org/10.1016/ j.chroma.2015.06.010
Kostanyan, A.E. and Erastov, A.A., Steady state preparative multiple dual mode counter-current chromatography: Productivity and selectivity. Theory and experimental verification, J. Chromatogr. A, 2015, vol. 1406, pp. 118–128. https://doi.org/10.1016/ j.chroma.2015.05.074
Chollet, S., Marchal, L., Meucci, J., Renault, J.-H., Legrand, J., and Foucault, A., Methodology for optimally sized centrifugal partition chromatography columns, J. Chromatogr. A, 2015, vol. 1388, pp. 174–183. https://doi.org/10.1016/j.chroma.2015.02.043
Kostanyan, A.E., Erastov, A.A., and Shishilov, O.N., Separation of liquid mixtures by dynamic countercurrent cyclic extraction, Theor. Found. Chem. Eng., 2015, vol. 49, no. 4, pp. 560–566. https://doi.org/10.1134/ S0040579515040119
Kostanyan, A.E., Analysis of the three-step cyclic process of countercurrent extraction, Theor. Found. Chem. Eng., 2015, vol. 49, no. 2, pp. 183–190. https://doi.org/ 10.1134/S0040579515020050
Friesen, J.B., Ahmed, S., and Pauli, G.F., Qualitative and quantitative evaluation of solvent systems for countercurrent separation, J. Chromatogr. A, 2015, vol. 1377, pp. 55–63. https://doi.org/10.1016/j.chroma.2014.11.085
Ito, Y., Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography, J. Chromatogr. A, 2005, vol. 1065, pp. 145–168. https://doi.org/10.1016/j.chroma.2004.12.044
Hewitson, P., Sutherland, I., Kostanyan, A.E., Voshkin, A.A., and Ignatova, S., Intermittent counter-current extraction—Equilibrium cell model, scaling and an improved bobbin design, J. Chromatogr. A, 2013, vol. 1303, pp. 18–27. https://doi.org/10.1016/ j.chroma.2013.06.023
Steblevskaya, N.I., Medkov, M.A., and Belobeletskaya, M.V., Extraction of the coordination compounds of rare earth elements with different ligands and its application to the synthesis of nanosized oxide composites, Vestn. Dal’nevost. Otd. Ross. Akad. Nauk, 2010, no. 5, pp. 67–74.
Peppard, D.F. and Wason, G.W., Liquid-liquid extraction of trivalent rare earths using acidic phosphonates as extractants, Rare Earth Research, Kleber, E.V., Ed., New York: Macmillan, 1961, pp. 37–50.
ACKNOWLEDGMENTS
This work was supported in part by the Russian Foundation for Basic Research (project no. 17-03-00263) and performed under a state order to the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, in the field of basic research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by M. Timoshinina
Rights and permissions
About this article
Cite this article
Belova, V.V., Martynova, M.M. Interphase Distribution of Lanthanide Salts in Multicomponent Aqueous–Organic Two-Phase Systems. Theor Found Chem Eng 53, 921–924 (2019). https://doi.org/10.1134/S0040579518050056
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579518050056