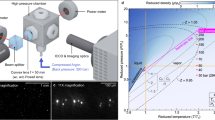
Abstract
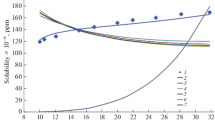
The adiabatic expansion of supercritical fluid solutions and solubility in pharmaceutical substance–carbon dioxide systems have been investigated. The solubility and average particle size of pharmaceutical substances depend on thermodynamic and geometric parameters of the process. Experimental data on the solubility of pharmaceutical substances in supercritical carbon dioxide have been gained, and empirical binary molecular interaction parameters for the Peng–Robinson equation have been derived. A numerical solution has been obtained for the unified model of nucleation and particle growth (in the drop theory approximation) in the expansion of a steady-state, two-dimensional, viscous, axisymmetric, compressible, supercritical carbon dioxide–pharmaceutical substance flow in a channel with a constant cross section and in a free jet. The correlation parameter of the condensation function, which characterizes the particle growth kinetics, has been determined.
Similar content being viewed by others
References
Valyashko, V.M., Phase equilibria involving supercritical fluids, Sverkhkrit. Flyuidy: Teor. Prakt., 2006, vol. 1, no. 1, pp. 10–26.
Tomasko, D.L., Li, H., Liu, D., Wingert, M.J., Lee, L.J., and Koelling, K.W., A review of CO2 applications in the processing of polymers, Ind. Eng. Chem. Res., 2003, vol. 42, pp. 6431–6456.
Alsoy, S. and Duda, J.L., Processing of polymers with supercritical fluids, Chem. Eng. Technol., 1999, vol. 22, pp. 971–973.
Mchugs, M.A. and Krukonis, V.J., Supercritical Fluid Extraction, Boston Butterworth–Heinemann, 1994.
Kendal, J.L., Canelas, D.A., Young, J.L., and de Simone, J.M., Polymerizations in supercritical carbon dioxide, Chem. Rev., 1999, vol. 99, pp. 543–563.
Hyatt, J.A., Liquid and supercritical carbon dioxide as organic solvents, Org. Chem., 1984, vol. 49, pp. 5097–5101.
Bagratashvili, V.N., Bogorodski, S.E., Konovalov, A.N., Kubyshkin, A.P., Novitski, A.A., Popov, V.K., Upton, C., and Howdle, S.M., Obtaining bioresorbable polymer microparticles using supercritical fluids, Sverkhkrit. Flyuidy: Teor. Prakt., 2007, vol. 2, pp. 53–60.
de Simone, J.M. and Guan, Z., Synthesis of fluoropolymers in supercritical carbon dioxide, Science, 1992, vol. 257, pp. 945–956.
Türk, M., Upper, G., and Hils, P., Formation of composite drug–polymer particles by co-precipitation during the rapid expansion of supercritical fluids, J. Supercrit. Fluids, 2006, vol. 39, pp. 253–263.
Agyarko, L., Nanopowder production: a comparison of several methods, Nat. Sci. Found. Res. Exp. Undergrad., 2004, vol. 9, pp. 35–63.
Kuznetsova, I.V, Gil’mutdinov, I.M., Khairutdinov, V.F., Mukhamadiev, A.A., Gumerov, F.M., and Sabirzyanov, A.N., Dispersion of pharmaceutical and polymer materials using supercritical fluids, Vestn. Kazan. Gos. Tekhnol. Univ., 2010, no. 2, pp. 321–328.
Gil’mutdinov, I.I., Gil’mutdinov, I.M., Kuznetsova, I.V., and Sabirzyanov, A.N., Solubility of methylparaben in supercritical carbon dioxide, Vestn. Kazan. Gos. Tekhnol. Univ., 2012, vol. 15, no. 1, pp. 108–110.
Gil’mutdinov, I.I., Kuznetsova, I.V., Ilalov, R.R., Gil’mutdinov, I.M., Mukhamadiev, A.A., and Sabirzyanov, A.N., Dispersion of ibuprofen by the rapid expansion of a supercritical solution, Vestn. Kazan. Gos. Tekhnol. Univ., 2011, vol. 14, no. 3, pp. 38–43.
Gil’mutdinov, I.I., Kuznetsova, I.V., and Sabirzyanov, A.N., Obtaining ibuprofen/polyethylene glycol 4000 and methylparaben/polyethylene glycol 4000 composite particles and study of their morphology and size, Vestn. Kazan. Gos. Tekhnol. Univ., 2013, vol. 16, pp. 96–98.
Gil’mutdinov, I.I., Gil’mutdinov, I.M., Kuznetsova, I.V., Safina, L.K., and Sabirzyanov, A.N., Experimental and theoretical study of methylparaben and ibuprofen solubility in supercritical carbon dioxide at T = 308 K, Vestn. Kazan. Technol. Univ., 2013, vol. 16, no. 18, pp. 57–59.
Gilmutdinov, I.I., Kuznetsova, I.V., Gilmutdinov, I.M., and Sabirzynov, A.N, Production and encapsulation of microand nanoparticles of pharmaceutical substances with the use of supercritical fluid, Proc. 10th Conf. on Supercritical Fluids and Their Applications, Napoli, 2013.
Gil’mutdinov, I.I., Kuznetsova, I.V., Gil’mutdinov, I.M., and Sabirzyanov, A.N., Mechanism of ibuprofen and methylparaben particle formation from supersaturated solutions in subcritical carbon dioxide, Vestn. Kazan. Technol. Univ., 2013, vol. 16, no. 9, pp. 83–87.
Gil’mutdinov, I.I., Kuznetsova, I.V., Gil’mutdinov, I.M., Mukhamadiev, A.A., and Sabirzyanov, A.N., Ibuprofen solubility in supercritical carbon dioxide, Sverkhkrit. Flyuidy: Teor. Prakt., 2012, vol. 7, no. 3, pp. 80–90.
Fishtine, S.H., The modified Lydersen method for predicting the critical constant of pure substances, Z. Phys. Chem., 1980, vol. 123, pp. 39–49.
Klincewicz, K.M. and Reid, R.C., Estimation of critical properties with group contribution methods, AIChE J., 1984, vol. 30, no. 1, pp. 137–142.
Gil’mutdinov, I.I., Gil’mutdinov, I.M., Kuznetsova, I.V., Khairutdinov, V.F., Yarullin, L.Yu., and Sabirzyanov, A.N., The mathematical description of the solubility of carbon dioxide in polyethylene glycol 4000 using the Sanchez–Lacombe equation of state, Vestn. Kazan. Gos. Technol. Univ., 2013, vol. 16, no. 18, pp. 60–62.
Kuznetsova, I.V., Gil’mutdinov, I.I., Gil’mutdinov, I.M., Mukhamadiev, A.A., and Sabirzyanov, A.N., Hydrodynamics and nucleation in a channel and in a free jet during the rapid expansion of a supercritical solution, Vestn. Kazan. Gos. Technol. Univ., 2012, vol. 15, no. 1, p. 111–117.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © I.M. Gil’mutdinov, I.I. Gil’mutdinov, I.V. Kuznetsova, A.N. Sabirzyanov, 2016, published in Teoreticheskie Osnovy Khimicheskoi Tekhnologii, 2016, Vol. 50, No. 1, pp. 18–31.
Rights and permissions
About this article
Cite this article
Gil’mutdinov, I.M., Gil’mutdinov, I.I., Kuznetsova, I.V. et al. Comminution of pharmaceutical substances by the adiabatic expansion of supercritical fluid solutions. Theor Found Chem Eng 50, 15–27 (2016). https://doi.org/10.1134/S0040579516010061
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579516010061