Abstract
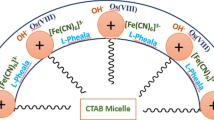
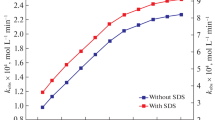
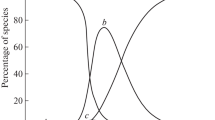
The kinetics of Os(VIII)-accelerated L-tryptophan (Trp) oxidation by hexacyanoferrate(III) in CTAB micellar medium were investigated by measuring the decline in absorbance at 420 nm, which corresponds to [Fe(CN)6]3–. By adjusting one variable at a time, the progression of the reaction has been inspected as a function of [OH–], ionic strength, [CTAB], [Os(VIII)], [Trp], [Fe(CN)\(_{6}^{{3 - }}\)], and temperature using the pseudo-first-order condition. The results show that [CTAB], [Trp], and [OH–] are the critical parameters with a discernible influence on reaction rate. The reaction rate is independent of the [Fe(CN)\(_{6}^{{3 - }}\)]; hexacyanoferrate(III) is merely used up to regenerate the Os(VIII) during the reaction. In the investigated concentration range of Os(VIII), as well as at lower [OH–] and [Trp], the reaction displays first-order kinetics with respect to [Os(VIII)], [OH–], and [Trp] but follows less than unit order at larger Trp and alkali concentrations. The linear increase in reaction rate with added electrolyte is indicative of a positive salt effect. CTAB significantly catalyzes the process, and once at a maximum, the rate remains almost constant as [CTAB] increases. The observed decrease in CTAB CMC could be attributed to reduced repulsion between the positive charge heads of surfactant molecules caused by the negatively charged [Fe(CN)6]3–, OH–, and [OsO5(OH)]3–.







Similar content being viewed by others
REFERENCES
T. Iioka, S. Takahashi, Y. Yoshida, Y. Matsumura, S. Hiraoka, and H. Sato, J. Comput. Chem. 40, 279 (2019).
R. M. Naik, A. Srivastava, A. K. Tiwari, S. B. S. Yaday, and A. K. Verma, J. Iran. Chem. Soc. 4, 63 (2007).
R. M. Naik, A. Srivastava, A. K. Verma, S. B. S. Yadav, R. Singh, and S. Prasad, Bioinorg. React. Mech. 6, 185 (2007).
R. O. Omondi, O. Stephen, S. O. Ojwach, and D. Jaganyi, Inorg. Chim. Acta 512, 119883 (2020).
A. Srivastava, R. M. Naik, J. Rai, and A. Asthana, Russ. J. Phys. Chem. A 95, 2545 (2021).
A. Singh and A. Singh, Prog. React. Kinet. Mech. 38, 105 (2013).
R. M. Naik, R. K. Tewari, P. K. Singh, A. K. Tiwari, and S. Prasad, Trans. Met. Chem. 30, 968 (2005).
S. Prasad, R. M. Naik, and A. Srivastava, Spectrochim. Acta, Part A 70, 958 (2008).
A. Srivastava, V. Sharma, A. Prajapati, N. Srivastava, and R. M. Naik, Chem. Chem. Technol. 13, 275 (2019).
A. Srivastava, V. Sharma, V. K. Singh, and K. Srivastava, J. Mex. Chem. Soc. 66, 57 (2022).
A. Gupta and A. Pandey, Ind. J. Sci. Res. 13, 66 (2017).
B. H. Asgha, H. M. Altas, and A. Fawzi, J. Saudi Chem. Soc. 21, 887 (2007).
E. Pandey, N. Grover, N. Kambo, and S. K. Uphadyay, Ind. J. Chem. A 43, 1183 (2004).
R. M. Naik, A. Srivastava, and A. K. Verma, Turk. J. Chem. 32, 495 (2008).
K. Sharanabasamma, M. A. Angadi, and S. M. Tuwar, Open Catal. J. 4, 1 (2011).
H. S. Singh, B. Singh, A. Gupta, and A. K. Singh, Oxid. Commun. 22, 146 (1999).
A. Goel and R. Sharma, J. Chem. Eng. Mater. Sci. 3, 1 (2012).
A. Nowdari, K. K. Adari, N. R. Gollapalli, and V. Parvataneni, Electron. J. Chem. 6, 93 (2009).
A. Goel and S. Sharma, Trans. Met. Chem. 35, 549 (2010).
J. Bagalkoti and S. T. Nandibewoor, Monatsh. Chem. 150, 1469 (2016).
S. A. Farokhi and S. T. Nandibewoor, Catal. Lett. 129, 207 (2009).
M. M. Al-Subu, Trans. Met. Chem. 26, 461 (2001).
M. M. Al-Subu, W. J. Jondi, A. A. Amer, M. Hannoun, and M. J. Musmar, Chem. Heterocycl. Compd. 39, 478 (2003).
D. F. J. Rani, F. J. M. Pushparaj, I. Alphonse, and K. S. Rangappa, Ind. J. Chem. B 41, 2153 (2002).
P. Sharma, R. Sailani, A. Meena, and C. L. Khandelwal, J. Chem. Res. 44, 295 (2020).
B. Das, B. Kumar, and W. Begum, Chem. Africa 5, 459 (2022).
M. A. Zahed, M. A. Matinvafa, and A. Azari, Discov. Water 5, 2 (2022).
D. C. Mohanambigai and D. Jenif, SPAST Abstr. 1, 1 (2021).
M. A. Karimi, M. A. Mozaheb, and A. Hatefi-Mehrjardi, J. Anal. Sci. Technol. 6, 1 (2015).
S. Shah, S. K. Chatterjee, and A. Bhattarai, J. Surfact. Deterg. 19, 201 (2016).
S. Tiwari, C. Mall, and P. P. Solanki, Surf. Interfaces 18, 100427 (2020).
S. K. Shah and A. Bhattarai, J. Chem., 4653092 (2020).
A. Rauf, M. K. Baloch, A. Khan, Z. Khan, and S. Rauf, J. Chil. Chem. Soc. 61, 3013 (2016).
R. C. Acharya, N. K. Saran, S. R. Rao, and M. N. Das, Int. J. Chem. Kinet. 14, 143 (1982).
S. K. Upadhyay, Int. J. Chem. Kinet. 15, 669 (1983).
P. J. Timy, S. T. Nandibewoor, and S. M. Tuwar, J. Sulf. Chem. 27, 25 (2006).
S. M. Zourab, E. M. Ezzo, H. J. El-Aila, and J. K. Salem, J. Disper. Sci. Technol. 24, 67 (2003).
A. Srivastava, Manjusha, N. Srivastava, and R. M. Naik, J. Mex. Chem. Soc. 67, 46 (2023).
L. Hadi, A. J. O. Richard, and E. R. Gavin, J. Am. Soc. Mass Spectrm. 15, 65 (2004).
P. L. Domingo, B. A. Garc, and J. M. Leal, Can. J. Chem. 68, 228 (1990).
S. Chowdhury, A. Rakshit, A. Acharjee, A. Ghosh, K. Mahali, and B. Saha, Tenside Surfact. Deterg. 57, 298 (2020).
S. Chowdhury, A. Rakshit, A. Acharjee, A. Ghosh, K. Mahali, and B. Saha, J. Mol. Liq. 290, 111247 (2019).
S. M. Tuwar, S. T. Nandibewoor, and J. R. Raju, Ind. J. Chem. A 30, 158 (1991).
H. B. Billalli, K. Sharanabasamma, and S. M. Tuwar, Prog. React. Kinet. Mech. 35, 347 (2010).
S. Dubey, N. Sharma, and C. L. Khandelwal, Trans. Met. Chem. 28, 176 (2003).
R. M. Naik and B. Kumar, J. Disp. Sci. Technol. 33, 647 (2012).
M. M. Graciani, M. A. Rodríguez, and M. L. Moyá, Int. J. Chem. Kinet. 29, 377 (1997).
C. A. Bunton, F. Nome, F. H. Quina, and L. S. Romsted, Acc. Chem. Res. 24, 357 (1991).
L. Brinchi, P. D. Profio, R. Germani, G. Savelli, M. Tugliani, and C. A. Bunton, Langmuir 16, 10101 (2000).
P. Lopez-Cornejo, J. D. Mozo, E. Roldán, M. Domínguez, and F. Sanchez, Chem. Phys. Lett. 352, 33 (2002).
D. Piszkiewicz, J. Am. Chem. Soc. 99, 7695 (1977).
P. K. Sen, N. Gani, and B. Pal, Ind. Eng. Chem. Res. 52, 2803 (2013).
A. Acharjee, A. Rakshit, S. Chowdhury, S. Malik, M. K. Barman, M. A. Ali, and B. Saha, J. Mol. Liq. 277, 360 (2019).
A. Ghosh, P. Das, D. Saha, P. Sar, S. K. Ghosh, and B. Saha, Res. Chem. Intermed. 42, 2619 (2016).
R. Jimenez, E. Bueno, I. Cano, and E. Corbacho, Int. J. Chem. Kinet. 26, 627 (2004).
Funding
We did not receive any specific grant for this research from any funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Srivastava, A., Srivastava, N. & Srivastava, K. Kinetic and Mechanistic Investigation of Os(VIII)-Catalyzed L-Tryptophan Oxidation by Hexacyanoferrate(III) in CTAB Micellar Medium. Russ. J. Phys. Chem. 97, 2932–2941 (2023). https://doi.org/10.1134/S0036024423130022
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024423130022