Abstract
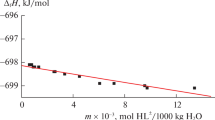
The enthalpies of dissolution of crystalline glycyl-L-tyrosine in water and in aqueous potassium hydroxide at 298.15 К were determined by the direct calorimetric method. The measurements were performed on a calorimetric unit with automatic recording of the temperature–time curve. The standard enthalpies of combustion and formation of glycyl-L-tyrosine were calculated by the additive group method based on group systematics with classification of fragments of the type of Benson classification, with the effect of the initial environment for atoms included in calculation. The standard enthalpies of formation of dipeptide and the products of its dissociation in aqueous solution were calculated.

Similar content being viewed by others
REFERENCES
A. V. Gumen, A. A. Butyugov, P. G. Nazarov, and V. V. Malinin, Byul. Eksp. Biol. Med. 131, 314 (2001).
N. I. Chalisova, A. N. Zakutskii, A. I. Aniskina, et al., Med. Akad. Zh. 6 (3), 57 (2006).
I. G. Kozlov, N. K. Gorina, and A. N. Cheredeeva, Immunologiya, No. 2, 45 (1997).
E. A. Markevicheva, S. V. Kuptsova, and L. D. Rumin, Vopr. Med. Khim. 48, 570 (2002).
D. M. Miller, D. de Silva, L. Pickart, and S. D. Aust, Adv. Exp. Med. Biol. 264, 79 (1990).
P. P. Korostelev, Preparation of Solutions for Chemical Analysis (Akad. Nauk SSSR, Moscow, 1962) [in Russian].
A. I. Lytkin, V. P. Barannikov, V. G. Badelin, and O. N. Krutova, J. Therm. Anal. Calorim. 139, 3683 (2020).https://doi.org/10.1007/s10973-019-08604-y
W. B. Parcker, Thermal Properties of Aqueous Uni-Univalent Electrolytes (NSRDS-NBS, Washington, DC, 1965), No. 2, p. 342.
A. V. Volkov, O. Yu. Platonycheva, O. N. Krutova, and V. G. Gradusov, Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol. 52 (4), 7 (2009).
L. A. Kochergina, V. G. Badelin, O. N. Krutova, A. V. Volkov, and K. V. Damrina, Russ. J. Phys. Chem. A 89, 1223 (2015).
V. P. Vasil’ev, V. A. Borodin, and S. B. Kopnyshev, Russ. J. Phys. Chem. A 65, 14 (1991).
A. V. Takhistov and D. A. Ponomarev, Organic Mass Spectrometry (VVM, St. Petersburg, 2002) [in Russian].
T. Kiss and Z. Szucs, J. Chem. Soc., Dalton Trans. 2, 2443 (1986).
V. P. Vasil’ev, Thermodynamical Properties of Electrolyte Solutions (Vysshaya Shkola, Moscow, 1982) [in Russian].
Thermal Properties of Individual Substances, Ed. by V. P. Glushko (VINITI, Moscow, 1965–1971), Vol. 3 [in Russian].
V. G. Badelin, E. Yu. Tyunina, and G. N. Tarasova, Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol. 50 (9), 76 (2007).
A. I. Lytkin, V. V. Chernikov, O. N. Krutova, and E. D. Krutova, Russ. J. Phys. Chem. A 94, 2569 (2020).
A. I. Lytkin, V. V. Chernikov, O. N. Krutova, and E. D. Krutova, Russ. J. Phys. Chem. A 94, 1342 (2020).
A. I. Lytkin, V. V. Chernikov, O. N. Krutova, K. V. Damrina, and I. A. Skvortsov, Russ. J. Phys. Chem. A 90, 735 (2016).
L. A. Kochergina, V. G. Badelin, A. I. Lytkin, et al., Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol. 57 (8), 11 (2014).
A. I. Lytkin, V. V. Chernikov, O. N. Krutova, and P. D. Krutov, Russ. J. Phys. Chem. A 94, 883 (2020).
A. I. Lytkin, V. V. Chernikov, and O. N. Krutova, Russ. J. Phys. Chem. A 90, 1530 (2016).
A. I. Lytkin, V. V. Chernikov, O. N. Krutova, I. A. Skvortsov, and A. S. Korchagina, Russ. J. Phys. Chem. A 90, 1782 (2016).
Funding
This study was performed at the Research Institute of Thermodynamics and Kinetics of Chemical Processes, Ivanovo State University of Chemistry and Technology (ISUCT) (project no. FZZW-2023-0008). It was conducted using the resources of the ISUCT Multiaccess Center and supported by the Ministry of Science and Higher Education of Russia (grant no. 075-15-2021-671).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by L. Smolina
Rights and permissions
About this article
Cite this article
Krutova, O.N., Bazanov, M.I., Chernikov, V.V. et al. Standard Enthalpies of Formation of Glycyl-L-tyrosine and Its Dissociation Products in Aqueous Solutions. Russ. J. Phys. Chem. 97, 2362–2366 (2023). https://doi.org/10.1134/S0036024423110171
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024423110171