Abstract
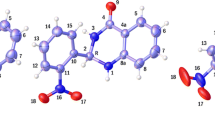
The thermodynamic stability of the axial (а) and equatorial (е) forms of the S- and R-enantiomers of 5,5,6-trihydroxy-6-methyldihydropyrimidine-2,4(1Н,3Н)-dione was studied by quantum-chemical methods. The equilibrium geometrical parameters and thermodynamic characteristics were determined by the DFT method using the TPSS functional combined with the 6-311+G(d,p) split-valence basis set including the d and p type polarization functions. The Chemcraft and VMD programs were used to visualize the geometrical structure. The most stable forms of 5,5,6-trihydroxy-6-methyldihydropyrimidine-2,4(1Н,3Н)-dione are Se and Re in both the gas phase and aqueous and organic (DMSO) media. The activation barrier of the rearrangement inside the ring is 21.22–24.93 kJ/mol depending on the medium.



Similar content being viewed by others
Notes
The data may be obtained from The Cambridge Crystallographic Data Centre at the address www.ccdc.cam.ac.uk/data_ reguest/cif).
REFERENCES
V. A. Myshkin and A. B. Bakirov, Oxymethyluracil (Essays on Experimental Pharmacology) (DAR, Ufa, 2001) [in Russian].
S. V. Jovanovic and M. G. Simic, J. Am. Chem. Soc. 108, 5968 (1986). https://doi.org/10.1021/ja00279a050
D. K. Hazra and S. Steenken, J. Am. Chem. Soc. 105, 4380 (1983). https://doi.org/10.1021/ja00351a042
J. A. Theruvathu, C. T. Aravindakumar, R. Flyunt, et al., J. Am. Chem. Soc. 123, 9007 (2001). https://doi.org/10.1021/ja0109794
C. von Sonntag, Int. J. Radiat. Appl. Instrum., Part C 30, 313 (1987). https://doi.org/10.1016/1359-0197(87)90101-9
M. Al-Sheikhly and C. von Sonntag, Z. Naturforsch. 38b, 1622 (1983). https://doi.org/10.1515/znb-1983-1214
T. Simandan, J. Sun, and T. A. Dix, Biochem. J. 335, 233 (1998). https://doi.org/10.1042/bj3350233
S. A. Grabovskiy, A. R. Abdrakhmanova, Yu. I. Murinov, and N. N. Kabal’nova, Curr. Org. Chem. 13, 1733 (2009). https://doi.org/10.2174/138527209789578081
S. A. Grabovskiy, I. G. Konkina, Yu. I. Murinov, and N. N. Kabal’nova, Curr. Org. Chem. 16, 1447 (2012). https://doi.org/10.2174/138527212800672619
S. P. Ivanov, I. G. Konkina, I. P. Baikova, et al., Chem. Heterocycl. Compd. 11, 1424 (2002). https://doi.org/10.1002/chin.200327138
S. F. Petrova, T. R. Nugumanov, A. N. Lobov, et al., Vestn. Bashkir. Univ. 21 (3), 626 (2016).
S. F. Petrova, S. S. Ostakhov, S. P. Ivanov, T. R. Nugumanov, Yu. I. Murinov, and S. L. Khursan, High Energy Chem. 52, 480 (2018). https://doi.org/10.1134/S0023119318060116
T. R. Nugumanov, S. P. Ivanov, Z. A. Starikova, and Y. I. Murinov, Mendeleev Commun. 18, 223 (2008). https://doi.org/10.1016/j.mencom.2008.07.020
S. F. Petrova, M. G. Il’ina, T. R. Nugumanov, et al., Izv. Ufim. Nauch. Tsentra RAN, No. 1, 112 (2020).
S. F. Petrova, T. R. Nugumanov, A. N. Lobov, L. V. Spirikhin, and S. P. Ivanov, J. Appl. Spectrosc. 89, 225 (2022). https://doi.org/10.1007/s10812-022-01347-z
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, et al., Gaussian 09 (Gaussian Inc., 2010).
G. A. Zhurko, Chemcraft. www.chemcraftprog.com.
J. Tao, J. P. Perdew, V. N. Staroverov, and G. E. Scuseria, Phys. Rev. Lett. 91, 146401 (2003). https://doi.org/10.1103/PhysRevLett.91.146401
K. K. Raghavachari, J. S. Binkley, R. Seeger, and J. A. Pople, J. Chem. Phys. 72, 650 (1980). https://doi.org/10.1063/1.438955
A. D. McLean and G. S. Chandler, J. Chem. Phys. 72, 5639 (1980). https://doi.org/10.1063/1.438980
J. Tomasi, B. Mennucci, and R. Cammi, Chem. Rev. 105, 2999 (2005). https://doi.org/10.1021/cr9904009
F. Floris and J. Tomasi, J. Comput. Chem. 10, 616 (1989). https://doi.org/10.1002/jcc.540100504
F. M. Floris, J. Tomasi, and J. L. P. Ahuir, J. Comput. Chem. 12, 784 (1991). https://doi.org/10.1002/jcc.540120703
R. A. Pierotti, Chem. Rev. 76, 717 (1976). https://doi.org/10.1021/cr60304a002
K. Ruud, T. Helgaker, K. L. Bak, P. Jørgensen, and H. J. Aa. Jensen, J. Chem. Phys. 99, 3847 (1993). https://doi.org/10.1063/1.466131
T. I. Lukmanov, G. S. Abdrakhimova, E. M. Khamitov, and S. P. Ivanov, Russ. J. Phys. Chem. A 86, 1104 (2012). https://doi.org/10.1134/S0036024412990010
V. M. Potapov, Stereochemistry (Khimiya, Moscow, 1988) [in Russian].
H. Günther, NMR Spectroscopy: An Introduction (Wiley, Chichester, 1980).
Funding
This study was performed under the government contract (no. 123011300044-5) of the Ministry of Science and Higher Education. The calculations were performed on a cluster supercomputer at the “Chemistry” Multiaccess Center, Ufa Institute of Chemistry, Ufa Federal Research Center, Russian Academy of Sciences, and “Agidel” Regional Multiaccess Center, Ufa Federal Research Center, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by L. Smolina
Rights and permissions
About this article
Cite this article
Khamitov, E.M., Petrova, S.F., Il’ina, M.G. et al. Theoretical Study of 5,5,6-Trihydroxy-6-methyldihydropyrimidine-2,4-dione Enantiomers. Russ. J. Phys. Chem. 97, 2275–2281 (2023). https://doi.org/10.1134/S003602442310014X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003602442310014X