Abstract
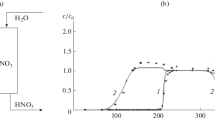
A dynamic model of the transfer and molecular sorption processes inside a sorption column is considered, based on the acid retardation of a gel anion exchanger. A three-layer model of a space filled with a solution is used to describe the process of keeping solution components inside nanosized pores in a multicomponent system. Allowance is made for the heterogeneity of the concentrations of molecules in the pores of the sorbent, caused by the forces acting on polar molecules from the sorption centers. The model allows calculation of changes in the concentrations of components over time inside the sorption column and, based on output concentration curves obtained experimentally, to determine characteristics of the process of holding molecules inside nanosized pores. Results from modeling are compared to experimental data on the purification of industrial extractive phosphoric acid.


Similar content being viewed by others
REFERENCES
M. J. Hatch and J. A. Dillon, I&EC Process Design Developm. 2, 253 (1963). https://doi.org/10.1021/i260008a001
N. B. Ferapontov, V. I. Gorshkov, Kh. T. Trobov, L. R. Parbuzina, O. T. Gavlina, and N. L. Strusovskaya, Russ. J. Phys. Chem. A 70, 840 (1996).
N. B. Ferapontov, V. I. Gorshkov, L. R. Parbuzina, et al., React. Funct. Polym. 66, 1749 (2006). https://doi.org/10.1016/j.reactfunctpolym.2006.08.005
V. A. Davankov, M. P. Tsyurupa, and N. N. Alexienko, J. Chromatogr., A 1100, 32 (2005). https://doi.org/10.1016/j.chroma.2005.09.007
V. Davankov, M. Tsyurupa, Z. Blinnikova, and L. Pavlova, J. Sep. Sci. 32, 64 (2009). https://doi.org/10.1002/jssc.200800449
A. N. Krachak, R. Kh. Khamizov, V. A. Poznukhova, et al., Sorbts. Khromatogr. Protsessy 11 (1), 77 (2011).
R. Kh. Khamizov, A. N. Krachak, A. N. Gruzdeva, et al., Geochem. Int. 54, 1221 (2016). https://doi.org/10.1134/S0016702916130085
R. Kh. Khamizov, A. N. Krachak, E. B. Podgornaya, and A. N. Gruzdeva, J. Anal. Chem. 74, 226 (2019). https://doi.org/10.1134/S1061934819030079
G. B. Sidelnikov, N. A. Tikhonov, R. K. Khamizov, and A. N. Krachak, Math. Models Comput. Simul. 5, 501 (2013). https://doi.org/10.1134/S2070048213060112
E. A. Glotova, N. A. Tikhonov, R. Kh. Khamizov, and A. N. Krachak, Mosc. Univ. Phys. Bull. 68, 65 (2013).
N. B. Ferapontov, V. I. Gorshkov, L. R. Parbuzina, H. T. Trobov, et al., React. Funct. Polym. 41, 213 (1999).
M. A. Kaznacheev, N. A. Tikhonov, and R. Kh. Khamizov, Sorbts. Khromatogr. Protsessy 21 (4), 547 (2021). https://doi.org/10.17308/sorpchrom.2021.21/3639
J. C. Brosheer, E. A. Lenfesty, and J. F. Anderson, J. Am. Chem. Soc. 76, 5951 (1954). https://doi.org/10.1021/ja01652a016
H. Galal-Gorchev, and W. Stumm, J. Inorg. Nucl. Chem. 25, 567 (1963). https://doi.org/10.1016/0022-1902(63)80243-2
B. P. Nikol’skii, O. N. Grigorov, M. E. Pozin, et al., Handbook of a Chemist, Vol. 3: Chemical Equilibrium and Kinetics of Solutions, Electrode Processes (Khimiya, Moscow, 1008) [in Russian].
Yu. Yu. Lur’e, Handbook on Analytical Chemistry (Khimiya, Moscow, 1971) [in Russian].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Kaznacheev, M.A., Tikhonov, N.A. & Khamizov, R.K. Determining Characteristics of a Model of Molecular Sorption, Based on an Example of the Separation of Components of Extractional Phosphoric Acid via “Retardation” on Ionites. Russ. J. Phys. Chem. 97, 1746–1750 (2023). https://doi.org/10.1134/S0036024423080095
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024423080095