Abstract
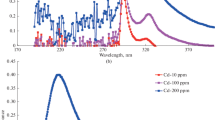
Results are described from studying the physicochemical properties of nanosized sorbents with a crystal structure of anatase and rutile, which are prepared by the high-energy milling of titania powders of respective modifications. Morphology, phase composition, and surface properties are studied by SEM, XRD, and XPS. The ξ-potential of sorbent suspensions as a function of pH is measured, and the point of zero charge is measured according to the shift in pH. It is found that milling for 8 h in an isopropyl alcohol medium results in a substantial increase in the number of crystallites with sizes of less than 10 nm; i.e., it greatly improves the sorption properties of titania with respect to ecotoxicants hexavalent chromium and trivalent arsenic ions, compared to the properties of the original material. The maximum amount of Cr(VI) is removed from aqueous solutions in acetate buffer medium at pH 5 and As(III) in hydrochloric acid medium at pH 2–3. A mechanism of adsorption is proposed.





Similar content being viewed by others
REFERENCES
B. Rivas, B. Urbano, M. Bryjal, et al., in Innovative Materials and Methods for Water Treatment: Solutions for Arsenic and Chromium Removal, Ed. by M. Bryak (Routledge, London, 2016).
P. A. Demina, A. M. Zybinskii, G. M. Kuz’micheva, et al., Crystallogr. Rep. 59, 430 (2014). https://doi.org/10.1134/S1063774514030079
B. Kurttekin, D. Özer, and N. Altuntaş Öztaş, Turk. J. Chem. 43, 492 (2019). https://doi.org/10.3906/kim-1808-6
G. M. Kuz’micheva, E. V. Savinkina, L. N. Obolenskaya, et al., Crystallogr. Rep. 55, 866 (2010). https://doi.org/10.1134/S1063774510050287
O. V. Mel’chakova, N. V. Pechishcheva, and A. D. Korobitsyna, Tsvet. Met., No. 1, 32 (2019). https://doi.org/10.17580/tsm.2019.01.05
M. Uzunova-Bujnova, D. Dimitrov, D. Radev, et al., Mater. Chem. Phys. 110, 291 (2008). https://doi.org/10.1016/j.matchemphys.2008.02.005
N. Pechishcheva, A. Korobitsyna, D. Ordinartsev, et al., Sep. Sci. Technol. 57, 180 (2022). https://doi.org/10.1080/01496395.2021.1891436
Z. Luan, E. M. Maes, P. A. W. van der Heide, et al., Chem. Mater. 11, 3680 (1999). https://doi.org/10.1021/cm9905141
V. V. Kaichev, Y. A. Chesalov, A. A. Saraev, et al., J. Catal. 338, 82 (2016). https://doi.org/10.1016/j.jcat.2016.02.022
M. Kosmulski, Adv. Colloid Interface Sci. 296, 102519 (2021). https://doi.org/10.1016/j.cis.2021.102519
S. Khalameida, E. Skwarek, W. Janusz, et al., Cent. Eur. J. Chem. 12, 1194 (2014). https://doi.org/10.2478/s11532-014-0568-5
M. Kosmulski, E. Mączka, and L. Ruchomski, J. Colloid Interface Sci. 533, 34 (2019). https://doi.org/10.1016/j.jcis.2018.08.050
C. Balan, I. Volf, and D. Bilba, Chem. Ind. Chem. Eng. Q. 19, 615 (2013). https://doi.org/10.2298/CICEQ120531095B
Z. Wei, K. Liang, Y. Wu, et al., J. Colloid Interface Sci. 462, 252 (2016). https://doi.org/10.1016/j.jcis.2015.10.018
M. E. Pena, G. P. Korfiatis, M. Patel, et al., Water Res. 39, 2327 (2005). https://doi.org/10.1016/j.watres.2005.04.006
A. L. Foster, G. E. Brown, and G. A. Parks, Environ. Sci. Technol. 32, 1444 (1998). https://doi.org/10.1021/es970846b
S. Bang, M. Patel, L. Lippincott, and X. Meng, Chemosphere 60, 389 (2005). https://doi.org/10.1016/j.chemosphere.2004.12.008
N. Pechishcheva, A. Belozerova, and K. Shunyaev, in Proceedings of 18th Israeli-Russian Bi-National Workshop on Optimization of the Composition, Structure, and Properties of Metals, Oxides, Composites, Nano and Amorphous Materials (Ein Bokek, 2019), p. 194.
ACKNOWLEDGMENTS
This work was performed on equipment at the Ural-M shared resource center and the Boreskov Institute of Catalysis. The authors thank A.V. Varaksin for his assistance in determining the specific surface area of titania.
Funding
This work was supported by the Russian Science Foundation, project no. 21-73-20039.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare they have no conflict of interest.
Additional information
Translated by M. Timoshinina
Rights and permissions
About this article
Cite this article
Ordinartsev, D.P., Pechishcheva, N.V., Valeeva, A.A. et al. Nanosized Titania for Removing Cr(VI) and As(III) from Aqueous Solutions. Russ. J. Phys. Chem. 96, 2408–2416 (2022). https://doi.org/10.1134/S0036024422110231
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024422110231