Abstract
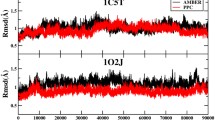
The folding and unfolding of proteins under exogenous perturbation is still a topic of great interest. People have conducted in-depth studies on the biological and health effects of electrostatic fields. In this study, we studied the conformational changes of trypsin inhibitor under different electric fields (low electric fields of 0.2 and 0.5–0.8 V/nm), and with the increase of the electric field, the conformational changes became more severe. Protease inhibitors are substances that can combine with enzymes to reduce the decomposition rate of substrates that enzymes act on. They are widely used in our lives and have important research value in food safety and biomedicine. Root mean square deviation (RMSD), dipole moment analysis and intermolecular hydrogen bond analysis were used to study the conformational changes of this protein.




Similar content being viewed by others
REFERENCES
S. Jain and E. Pirogova, in Proceedings of the IEEE Progress in Electromagnetics Research Symposium - Fall PIERS - FALL (2017), p. 1273.
P. Norouzi and R. Ghiasi, Physics (Amsterdam, Neth.) 118 (24), e1781272 (2020).
P. Norouzi, R. Ghiasi, and R. Fazaeli, Russ. J. Inorg. Chem. 65, 2053 (2021).
L. Astrakas, C. Gousias, and M. Tzaphlidou, J. Appl. Phys. 109 (9), 094702 (2011).
W.-H. Du, Y.-H. Han, F.-J. Huang, et al., Febs J. 274, 2596 (2007).
M. Breton, L. Delemotte, A. Silve, et al., J. Am. Chem. Soc. 134, 13938 (2012).
R. Day, B. J. Bennion, S. Ham, et al., J. Mol. Biol. 322, 189 (2002).
J. Wiedersich, S. Koehler, A. Skerra, et al., Proc. Natl. Acad. Sci. U. S. A. 105, 5756 (2008).
V. F. Ur’yash and N. Y. Kokurina, Russ. J. Phys. Chem. A 87, 1632 (2013).
C. M. Kelleher, J. A. O’mahony, A. L. Kelly, et al., Int. Dairy J. 85, 144 (2018).
F. Qian, J. Sun, D. Cao, et al., Korean J. Food Sci. Animal Resour. 37, 44 (2017).
M. L. Swicord, A. R. Sheppard, and Q. Balzano, J. Chem. Phys. 126, 091105 (2007);
J. Chem. Phys. 127 (11) (2007).
P. Marracino, F. Apollonio, M. Liberti, et al., J. Phys. Chem. B 117, 2273 (2013).
D. Roy, J. N. Cambre, and B. S. Sumerlin, Prog. Polym. Sci. 35, 278 (2010).
A. Singh, R. Lahlali, S. K. Vanga, et al., Int. J. Food Propert. 19, 1217 (2016).
V. A. Sirotkin and D. V. Korolev, Russ. J. Phys. Chem. 80, 929 (2006).
L. G. Astrakas, C. Gousias, and M. Tzaphlidou, J. Appl. Phys. 111 (7), 074702 (2012).
B. H. Vagadia, S. K. Vanga, A. Singh, et al., Innov. Food Sci. Emerg. Technol. 35, 9 (2016).
A. Budi, S. Legge, H. Treutlein, et al., Eur. Biophys. J. 33, 121 (2004).
N. J. English and D. A. Mooney, J. Chem. Phys. 126 (9), 2577 (2007).
W. L. Jorgensen, J. Chandrasekhar, J. D. Madura, et al., J. Chem. Phys. 79, 926 (1983).
H. J. C. Berendsen, J. P. M. Postma, W. F. van Gunsteren, et al., J. Chem. Phys. 81, 3684 (1984).
X. Wang, Y. Li, X. He, et al., J. Phys. Chem. A 118, 8942 (2014).
W. Kabsch and C. Sander, Biopolymers (1983).
S. S. Jain, A. Suresh, and E. Pirogova, J. Mol. Graph. Model. 103, 107799 (2021).
A. Budi, F. S. Legge, H. Treutlein, et al., J. Phys. Chem. B 112, 7916 (2008).
A. Budi, F. S. Legge, and H. Treutlein, J. Phys. Chem. B (2005).
V. Daneshdoost, R. Ghiasi, and A. Marjani, J. Struct. Chem. 61, 1691 (2020).
Z. Kazemi, R. Ghiasi, and S. Jamehbozorgi, Adsorption 26, 905 (2019).
R. Ghiasi, M. V. Sofiyani, and R. Emami, Biointerface Res. Appl. Chem. 11, 12454 (2021).
Funding
This work was supported by National Natural Science Foundation of China (62105196) and Shanghai Sailing Program (17YF1407000).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Zheng, K., Ji, Mh., Chu, Fh. et al. The Effect of External Electric Field on the Conformational Integrity of Trypsin Inhibitor: A Molecular Model Study. Russ. J. Phys. Chem. 96, 2533–2540 (2022). https://doi.org/10.1134/S0036024422110103
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024422110103