Abstract
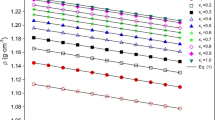
The viscosity and density of water and water-ethanol mixtures containing different concentrations of MgSO4 were measured in the temperature range of 303.15 to 323.15 K with a difference of 5.0 K. The viscosity increased with an increase in the concentration of MgSO4 and ethanol in the solution while it decreased with the increase of temperature. The density of the solutions increased with an increase in the concentration of MgSO4 while it decreased with an increase in the concentration of ethanol in the solution. The ionic interactions were evaluated from the straight-line plots of the Jones–Dole equation; ηsp/C1/2 = A + BC1/2. The ion-solvent interactions decreased while the ion-ion interactions increased with the increase of temperature. A negative dB/dT confirmed the structure-making behavior of Mg2+ ions in water and water-ethanol mixtures. The plots of the Moulik equation were linear with good correlation coefficients and the fluidity parameters were found to be strongly dependent on the temperature. The activation energy (Ea) was negative which supports the structure-making behavior of Mg2+ ions. The thermodynamic parameters provided evidence that the interaction of MgSO4 with water and water-ethanol mixture is an exothermic and structure-promoting process.










Similar content being viewed by others
REFERENCES
B. B. Danahy, D. L. Minnick, and M. B. Shiflett, Fermentation 4, 72 (2018).
A. Maitra and S. Bagchi, J. Mol. Liq. 137, 131 (2008).
A. Ben-Naim, J. Phys. Chem. 93, 3809 (1989).
C. Reichardt, Chem. Rev. 94, 2319 (1994).
M. S. Ghoraishi, J. E. Hawk, A. Phani, M. F. Khan, and T. Thundat, Sci. Rep. 6, 23966 (2016).
X. Li, X. Wang, M. D. Passaro, N. Spinelli, and B. Apicella, J. Am. Soc. Mass Spectrom. 26, 1665 (2015).
F. Frank and D. J. Ives, Q. Rev. Chem. Soc. 20, 1 (1966).
R. K. Schofield and E. K. Rideal, Proc. R. Soc. London, Ser. A 109, 57 (1925).
R. K. Schofield and E. K. Rideal, Proc. R. Soc. London, Ser. A 110, 167 (1926).
H. Li, C. Song, L. Xu, and G. Liu, Russ. J. Phys. Chem. A 94, 1356 (2020).
T. S. Jyostna, B. Satheesh, D. Sreenu, et al., Russ. J. Phys. Chem. A 93, 278 (2019).
X. Pan, D. Li, M. Guoa, et al., Russ. J. Phys. Chem. A 94, 2574 (2020).
M. Aftabuzzaman, M. M. Islam Nasiruddin, F. R. Rima, et al., J. Chem. Thermodyn. 96, 181 (2016).
Z. R. Master and N. I. Malek, J. Mol. Liq. 196, 120 (2014).
S. Thirumaran and K. Sathish, Res. J. Chem. Sci. 1, 63 (2011).
S. Seki, K. Hayamizu, S. Tsuzuki, et al., J. Electrochem. Soc. 165, 542 (2018).
N. Sundaramurthy, B. Rajamannan, and S. Rajalakshmi, Int. J. Curr. Res. 3, 89 (2010).
M. N. Roy, D. Ekka, and R. Dewan, Acta Chim. Slov. 58, 792 (2011).
L. C. Heda, R. Sharma, S. R. Mosalpuri, and P. B. Chaudhari, Ecl. Quim. Sao Paulo 35, 23 (2010).
K. Rajagopal and S. S. Jayabalakrishnan, Chi. J. Chem. Eng. 17, 796 (2009).
H. Jaka, B. Marija, K. Cveto, and R. Darja, J. Sol. Chem. 37, 1329 (2008).
T. Zamir and A. Khan, J. Chem. Soc. Pakist. 27, 130 (2005).
A. Ali, A. K. Nain, N. Kumar, and M. Ibrahim, Proc. Indian Acad. Sci. (Chem. Sci.) 114, 495 (2002).
A. G. Shankarwar, V. A. Shelke, and S. G. Shankarwar, Adv. Appl. Sci. Res. 2, 426 (2011).
K. Jayalaksmi and K. S. Reddy, Proc. Indian Acad. Sci. (Chem. Sci.) 94, 457 (1985).
C. S. Solanki, S. Tripathy, M. Tripath, and U. N. Dash, El.-J. Chem. 7, 223 (2010).
A. R. Khan, Shama, R. Saeed, and F. Uddin, J. Appl. Sci. Environ. Mgt. 9, 15 (2005).
D. R. Rohindra, R. A. Lata, and R. K. Coll, Eur. J. Phys. 33, 1457 (2012).
J. D. Pandey and Y. Akhtar, Proc. Indian Acad. Sci. (Chem. Sci.) 109, 289 (1997).
M. R. Schroeder, B. E. Poling, and D. B. Manley, J. Chem. Eng. Data 27, 256 (1982).
Y. Takiguchi and M. Uematsu, J. Chem. Thermodyn. 28, 7 (1996).
D. Swiatla-Wojcik, A. Pabis, and J. Szala, Cent. Eur. J. Chem. 6, 555 (2008).
S. L. Wallen, B. J. Palmer, B. C. Garrett, and C. R. Yonker, J. Phys. Chem. 100, 3959 (1996).
G. Jones and M. Dole, J. Am. Chem. Soc. 51, 2950 (1929).
R. Saeed, S. Masood, and S. M. S. Nadeem, Int. J. Chem. 4, 28 (2012).
K. H. Kapandis and A. P. Hiray, Chem. Sci. Trans. 2, 485 (2013).
R. Saeed, F. Uddin, S. Masood, and N. Asif, J. Mol. Liq. 146, 112 (2009).
H. Ahmed, Y. Khan, S. S. Shah, A. Mumtaz, M. A. Razi, and E. A. Mukhtar, J. Chem. Soc. Pakist. 26, 5 (2004).
M. Kaminsky, Discuss. Faraday Soc. 24, 171 (1957).
H. Peng and A. V. Nguyen, J. Mol. Liq. 263, 109 (2018).
M. Kumar, S. Kant, and D. Kaushal, Z. Phys. Chem. 233, 255 (2018).
S. P. Musale, P. S. Babalsure, and D. D. Pawar, J. Mol. Liq. 319, 114197 (2020).
S. Thawarkar, N. D. Khupse, and A. Kumar, Phys. Chem. Chem. Phys. 17, 475 (2015).
S. P. Moulik, J. Phys. Chem. 72, 4682 (1968).
B. F. Aderson and K. O. Ipinmoroti, Pakist. J. Sci. Ind. Res. 42, 325 (1999).
E. R. Nightingale, Jr. and R. F. Benck, J. Phys. Chem. 63, 1777 (1959).
E. A. Masimov, B. G. Pashayev, and H. Sh. Hasanov, Russ. J. Phys. Chem. A 93, 988 (2019).
O. Y. Samoilov, Discuss. Faraday Soc. 24, 141 (1957).
E. R. Nightingale, Jr., J. Phys. Chem. 63, 1381 (1959).
F. J. Rubio-Hernandez, A. I. Gomez-Merino, R. Delgado-Garcia, and N. M. Paez-Flor, Powder Technol. 308, 318 (2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that he has no conflicts of interest.
Rights and permissions
About this article
Cite this article
Saqib Nadeem, S.M. Viscometric Study of Ionic Interactions of MgSO4 in Water and Water–Ethanol Mixtures at Different Temperatures. Russ. J. Phys. Chem. 96, 849–859 (2022). https://doi.org/10.1134/S0036024422040306
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024422040306