Abstract
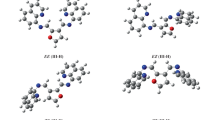
A novel Schiff base derivative, 4,4'-(propane-1,3-diylbis(azanylylidene))bis(naphthalen-1(4H)-one) (PAN), was synthesized from the reaction of 1,4-naphthoquinone with 1,3-diaminopropane. Synthesized substance was characterized by using 1H and 13C NMR, FT-IR, UV–Vis spectroscopies and elemental analyses. Optimized geometry, geometric parameters, molecular electrostatic potential (MEP) diagram and vibrational wavenumbers of the PAN were calculated by using Density Functional Theory (DFT) at B3LYP/6-311G(d,p) level. 1H- and 13C-NMR chemical shifts were theoretically obtained using the gauge independent atomic orbital (GIAO) method with mentioned level of theory. Furthermore electronic transitions, frontier molecular orbital energies (FMOs) such as HOMO and LUMO were also calculated by time-dependent DFT (TD-DFT) approach. Theoretically calculated spectroscopic data were found to be quite compatible with those obtained experimentally.









Similar content being viewed by others
REFERENCES
M. Tisler, in Advances in Heterocyclic Chemistry, Ed. by A. Katritzky (Academic, London, 1989).
J. Elguero, A. M. S. Silva, and A. C. Tomé, in Modern Heterocyclic Chemistry, Ed. by J. Alvarez-Builla, J. J. Vaquero, and J. Barlueng (Wiley-VCH, Weinheim, 2011), Vol. 2.
T. J. Monks, R. P. Hanzlik, G. M. Cohen, D. Ross, and D. G. Graham, Toxicol. Appl. Pharm. 112, 2 (1992).
E. Shor and D. S. Perlin, Plos Pathog. 11, e1004668 (2015).
S. T. Micek, R. G. Wunderink, M. H. Kollef, C. Chen, J. Rello, J. Chastre, M. Antonelli, T. Welte, B. Clair, H. Ostermann, E. Calbo, A. Torres, F. Menichetti, G. E. Schramm, and V. Menon, Crit. Care 19, 219 (2015).
A. R. Surrey and H. F. Hammer, J. Am. Chem. Soc. 68, 113 (1946).
J. Wiesner, R. Ortmann, H. Jomaa, and M. Schlitzer, Angew. Chem. Int. Ed. 42, 5274 (2003).
D. C. Leysen, M. Q. Zhang, A. Haemers, and W. Bollaert, Pharmazie 46, 485 (1991).
J. Duffour, S. Gourgou, F. Desseigne, C. Debrigode, L. Mineur, F. Pinguet, S. Poujol, P. Chalbos, F. Bressole, and M. Ychou, Cancer Chemother. Pharm. 60, 283 (2007).
M. Takamura, K. Funabashi, M. Kanai, and M. Shibasaki, J. Am. Chem. Soc. 123, 6801 (2001).
B. S. Tovrog, D. J. Kitko, and R. S. Drago, J. Am. Chem. Soc. 98, 5144 (1976).
P. S. Dixit and K. Srinivason, Inorg. Chem. 27, 4507 (1988).
P. E. Aranha, M. P. dos Santos, S. Romera, and E. R. Dockal, Polyhedron 26, 1373 (2007).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, et al., Gaussian 09, Revision E.01 (Gaussian Inc., Wallingford CT, 2016).
R. Dennington, T. A. Keith, and J. M. Millam, GaussView, Revision 5.0.9 (Semichem Inc., Shawnee Mission, KS, 2009).
A. D. Becke, J. Chem. Phys. 98, 5648 (1993).
C. Lee, W. Yang, and R. G. Parr, Phys. Rev. B 37, 785 (1988).
K. Wolinski, J. F. Himton, and P. Pulay, J. Am. Chem. Soc. 112, 8251 (1990).
N. M. O’Boyle, A. L. Tenderholt, and K. M. Langner, J. Comp. Chem. 29, 839 (2008).
M. C. Pham, B. Piro, E. A. Bazzaoui, M. Hedayatullah, J. C. Lacroix, P. Novák, and O. Haas, Synth. Met. 92, 197 (1998).
A. Satheshkumar and K. P. Elango, RSC Adv. 3, 1502 (2013).
K. H. Hartmann and T. Troll, Tetrahedron 51, 4655 (1995).
N. R. Sperandeo, M. M. de Bertorello, and M. C. Briñón, J. Pharm. Sci. 83, 332 (1994).
L. J. Bellamy, The Infrared Spectra of Complex Molecules, 3rd ed. (Wiley, New York, 1975).
N. B. Colthup, L. H. Daly, and E. Wiberley, Introduction to Infrared and Raman Spectroscopy (Academic, New York, 1964).
K. Fukui, Science (Washington, DC, U. S.) 218, 747 (1982).
R. Pearson, J. Org. Chem. 54, 1423 (1989).
R. G. Pearson, Proc. Natl. Acad. Sci. U. S. A. 83, 8440 (1986).
P. Politzer, J. S. Murray, and M. C. Concha, Int. J. Quantum Chem. 88, 19 (2002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dede, B., Aysan, Ö. & Yildirim, F. Synthesis, Spectroscopic Properties, and DFT Calculations of Novel Naphthoquinone Based Diimine Molecule. Russ. J. Phys. Chem. 95 (Suppl 1), S99–S108 (2021). https://doi.org/10.1134/S0036024421140053
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024421140053