Abstract
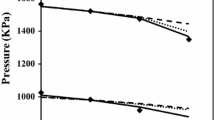
A comparison is made between theoretical and experimental studies of the position of eutectics in two-component systems consisting of tetrachlorethylene and n-alkanes with 10 to 20 carbon atoms. Data on the composition of the eutectics, their temperatures of melting, and the coefficients of activity of each component are calculated in three ways: using experimental data, the UNIFAC procedure, and a modified UNIFAC Dortmund procedure. The equilibria of liquid–solid phases are studied theoretically using Schröder’s equation and the UNIFAC and modified UNIFAC Dortmund procedure. Experimental data were obtained via DTA. Relative deviations of the calculated melting temperatures and compositions of the eutectics from the corresponding experimental data are reported.




Similar content being viewed by others
REFERENCES
U. Weidlich and J. Gmehling, Ind. Eng. Chem. Res., No. 26, 1372 (1987).
K. Paduszyński and U. Domańska, Fluid Phase Equilib. 353, 115 (2013). https://doi.org/10.1016/j.fluid.2013.06.025
S. Nebig and J. Gmehling, Fluid Phase Equilib. 294, 206 (2010). https://doi.org/10.1016/j.fluid.2010.02.010
D. Costa do Nascimento, N. D. D. Carareto, A. M. Barbosa Neto, et al., Fuel 281, 118717 (2020). https://doi.org/10.1016/j.fuel.2020.118717
T. Fornari, R. P. Stateva, F. J. Señorans, et al., J. Supercrit. Fluids 46, 245 (2008). https://doi.org/10.1016/j.supflu.2007.11.017
I. Bahadur, A. Pal, J. Gmehling, et al., J. Chem. Thermodyn. 90, 92 (2015). https://doi.org/10.1016/j.jct.2015.06.023
S. Nebig, V. Liebert, and J. Gmehling, Fluid Phase Equilib. 277, 61 (2009). https://doi.org/10.1016/j.fluid.2008.11.013
S. Nebig, R. Bölts, and J. Gmehling, Fluid Phase Equilib. 258, 168 (2007). https://doi.org/10.1016/j.fluid.2007.06.001
M. Klauck, R. Silbermann, R. Metasch, et al., Fluid Phase Equilib. 314, 169 (2012). https://doi.org/10.1016/j.fluid.2011.09.010
J. A. González, I. García de la Fuente, and J. C. Cobos, Fluid Phase Equilib. 154, 11 (1999). https://doi.org/10.1016/S0378-3812(98)00421-X
J. A. González, I. García de la Fuenté, and J. C. Cobos, Fluid Phase Equilib. 168, 31 (2000). https://doi.org/10.1016/S0378-3812(99)00326-X
M. D. Robustillo, L. C. B. A. Bessa, and P. de Alcantara Pessôa Filho, Fluid Phase Equilib. 530, 112874 (2021). https://doi.org/10.1016/j.fluid.2020.112874
F. Rabhi, T. D. Pietro, F. Mutelet, and H. Sifaoui, J. Mol. Liq. 325, 115231 (2021).
L. P. Zini, P. B. Staudt, and R. de P. Soares, Fluid Phase Equilib., 112942 (2021).
K. Xin, I. Roghair, F. Gallucci, and M. van Sint Annaland, J. Mol. Liq. 325, 115227 (2021). https://doi.org/10.1016/j.molliq.2020.115227
X. Zhang, M. Li, Y. Hu, Z. Liu, and S. Mo, Chin. J. Chem. Eng. (2020). https://doi.org/10.1016/j.cjche.2020.11.019
B. Bazooyar, F. Shaahmadi, M. A. Anbaz, and A. Jomekian, J. Mol. Liq. 322, 114972 (2021). https://doi.org/10.1016/j.molliq.2020.114972
S. Balchandani and R. Singh, J. Mol. Liq. 324, 114713 (2020). https://doi.org/10.1016/j.molliq.2020.114713
T. I. Farouk, S. H. Won, and F. L. Dryer, Proc. Combust. Inst. (2020). https://doi.org/10.1016/j.proci.2020.06.200
C. L. Silveira, A. C. Galvão, W. S. Robazza, and J. V. T. Feyh, Fluid Phase Equilib., 112970 (2021). https://doi.org/10.1016/j.fluid.2021.112970
M. D. Robustillo, L. C. B. Almeida Bessa, P. de Alcantara Pessôa Filho, Fluid Phase Equilib. 530, 112874 (2021). https://doi.org/10.1016/j.fluid.2020.112874
G. P. Assis, R. H. L. Garcia, S. Derenzo, and A. Bernardo, J. Mol. Liq. 323, 114617 (2021). https://doi.org/10.1016/j.molliq.2020.114617
P. Sikorski, A. C. Huerga, J. A. González, M. Królikowski, and T. Hofman, J. Mol. Liq. 318, 114265 (2020). https://doi.org/10.1016/j.molliq.2020.114265
T. Zhang, A. Li, X. Xu, et al., J. Chem. Thermodyn. 152, 106284 (2021). https://doi.org/10.1016/j.jct.2020.106284
Z. Wang, S. Liu, Y. Jiang, et al., Green Energy Environ. (2020). https://doi.org/10.1016/j.gee.2020.12.021
S. Liu, Z. Wang, R. Zhu, et al., Green Energy Environ. (2020). https://doi.org/10.1016/j.gee.2020.12.022
Y. Jiang, Z. Wang, Z. Lei, and G. Yu, Chem. Eng. Sci. 230, 116186 (2021). https://doi.org/10.1016/j.ces.2020.116186
E. V. Dorokhina, Cand. Sci. (Chem.) Dissertation (Saratov, 2012).
I. K. Garkushin, E. M. Dvoryanova, and O. U. Aphanasieva, in Proceedings of the 17th International Conference of Chemical Thermodynamica in Russia (2009), Vol. 1, p. 337.
E. V. Dorokhina, A. V. Kolyado, and Yu. V. Moshchenskii, in Chemistry, Collection of Articles (Samar. Gos. Tekh. Univ., Samara, 2009), p. 40 [in Russian].
E. V. Dorokhina, A. V. Kolyado, and I. K. Garkushin, Butler. Soobshch. 25 (8), 51 (2011).
E. V. Dorokhina and A. V. Kolyado, in Proceedings of the 9th International Kurnakov Workshop on Physicochemical Analysis (Perm’, 2010), p. 95.
E. V. Dorokhina, A. V. Kolyado, and I. K. Garkushin, in Chemistry, Collection of Articles (Samar. Gos. Tekh. Univ., Samara, 2009, p. 47 [in Russian].
E. V. Dorokhina, A. V. Kolyado, and I. K. Garkushin, in Proceedings of the 20th Russia Youth Conference on Problems of Theoretical and Experimental Chemistry, Dedicated to 90th Anniversary of Gorky Ural State Univ., Ekaterinburg, April 20–24, 2010 (Ural. Univ., Yekaterinburg, 2010), p. 319.
E. V. Dorokhina, A. V. Kolyado, and I. K. Garkushin, Izv. Sarat. Univ., Ser. Khim. Biol. Ekol. 11 (1), 31 (2011).
E. V. Dorokhina, A. V. Kolyado, I. K. Garkushin, et al., Bashkir. Khim. Zh. 17 (3), 30 (2010).
I. K. Garkushin, E. V. Dorokhina, and A. V. Kolyado, Butler. Soobshch. 16 (3), 41 (2009).
I. K. Garkushin, A. V. Kolyado, and E. V. Dorokhina, Calculation and Study of Phase Equilibria in Binary Systems of Organic Substances (UrO RAN, Yekaterinburg, 2011), p. 191 [in Russian].
A. G. Morachevskii, N. A. Smirnova, E. M. Piotrovskaya, et al., Thermodynamics of Liquid-Vapour Equilibrium (Khimiya, Leningrad, 1989), p. 344 [in Russian].
J. Gmehling, J. Li, and M. Schiller, Ind. Eng. Chem. Res. 32, 178 (1993).
Gmehling J, J. Lohmann, A. Jakob, J. Li, and R. Joh, Ind. Eng. Chem. Res. 37, 4876 (1998).
J. Gmehling, R. Wittig, J. Lohmann, and R. Joh, Ind. Eng. Chem. Res. 41, 1678 (2002).
R. Wittig, J. Lohmann, R. Joh, Horstmann, and J. Gmehling, Ind. Eng. Chem. Res. 40, 5831 (2001).
J. Lohmann, R. Joh, and J. Gmehling, Ind. Eng. Chem. Res. 40, 957 (2001).
J. Lohmann and J. Gmehling, J. Chem. Eng. Jpn., No. 34, 43 (2001).
R. Wittig, J. Lohmann, and J. Gmehling, AIChE J. 49, 530 (2003).
A. Jakob, H. Grensemann, J. Lohmann, and J. Gmehling, Ind. Eng. Chem. Res. 45, 7924 (2006).
T. Hector and J. Gmehling, Fluid Phase Equilib. 371, 82 (2014).
D. Constantinescu and J. Gmehling, J. Chem. Eng. Data 61, 2738 (2016).
http://unifac.ddbst.de/modified-unifac.html. Accessed January 11, 2021.
E. S. Domalski and E. D. Hearing, J. Phys. Chem. Ref. Data 25, 1 (1996). https://doi.org/10.1063/1.555985
ACKNOWLEDGMENTS
The authors are grateful to S.I. Yakovleva for her help in preparing and formatting the article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Ivanov
Rights and permissions
About this article
Cite this article
Yakovlev, I.G., Garkushin, I.K. & Kolyado, A.V. Coefficients of Activity in Tetrachloroethylene–n-Alkane Systems. Russ. J. Phys. Chem. 95, 1990–1995 (2021). https://doi.org/10.1134/S0036024421100307
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024421100307