Abstract
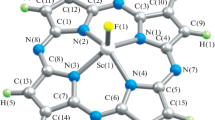
The molecular structures of the (6666) macrotetracyclic heteroligand chelates of M(III) (M = Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu) with the (NNNN)-donor-atom ligand phthalocyanine and fluoride anion were calculated using the density functional theory (DFT) in the OPBE/TZVP version. All these metal complexes have the structure of a slightly distorted tetragonal pyramid, where the M(III) complexing agent lies over its base consisting of nitrogen donor atoms. The values of the most important bond lengths and bond and non-bond angles in these complexes were given. All the six-membered metal chelate cycles in eight of these nine metal chelates are identical both in the sum of their bond angles and assortment; the only exception is the Mn(III) complex, in which these metallocycles are equal only in pairs. Pronounced electron density delocalization takes place over the entire macrocycle in each of these coordination compounds. The standard enthalpy, entropy, and Gibbs energy of formation of these compounds were also calculated.



Similar content being viewed by others
REFERENCES
K. Kasuda and M. Tsutsui, Coord. Chem. Rev. 32, 67 (1980).
A. L. Thomas, Phthalocyanines. Research and Applications (CRC, Boca Raton, FL, 1990).
W. Sliva and B. Mianovska, Trans. Met. Chem. 25, 491 (2000).
G. M. Mamardashvili, N. Z. Mamardashvili, and O. I. Koifman, Russ. Chem. Rev. 77, 59 (2008).
T. N. Lomova, Axially Coordinated Metalloporphyrins in Science and Practice (URSS-KRASAND, Moscow, 2018) [in Russian].
O. G. Khelevina and A. S. Malyasova, J. Porphyr. Phthalocyan. 23, 1251 (2019).
Kikuko Okada, Atsumi Sumida, Rie Inagaki, and Masahiko Inamo, Inorg. Chim. Acta. 392, 473 (2012).
C. Colomban, E. V. Kudric, P. Afanasiev, and A. B. Sorokin, J. Am. Chem. Soc. 136, 11321 (2014). https://doi.org/10.1021/ja505437h
J. W. Buchler and K. Rohbock, Inorg. Nucl. Chem. Lett. 8, 1073 (1972).
R. Guilard, P. Richard, M. El Borai, and E. Laviron, J. Chem. Soc., Chem Commun., No. 11, 516 (1980). https://doi.org/10.1039/C39800000516
C. Lecomte, J. Protas, P. Richard, et al., J. Chem. Soc., Dalton Trans., No. 2, 247 (1982). https://doi.org/10.1039/DT9820000247
P. A. Stuzhin, in Fluorine in Heterocyclic Chemistry, Ed. by V. G. Nenajdenko, Vol. 1, 5: Membered Heterocycles and Macrocycles (Springer, Heidelberg, 2014), p. 621.
I. A. Lebedeva (Yablokova), S. S. Ivanova, Y. A. Zhabanov, et al., J. Fluorine Chem. 214, 86 (2018).
D. V. Chachkov and O. V. Mikhailov, Russ. J. Inorg. Chem. 58, 174 (2013). https://doi.org/10.1134/S0036023613020186
D. V. Chachkov and O. V. Mikhailov, Russ. J. Inorg. Chem. 59, 218 (2014). https://doi.org/10.1134/S0036023614030024
O. V. Mikhailov and D. V. Chachkov, Russ. J. Inorg. Chem. 60, 1354 (2015). https://doi.org/10.1134/S003602361511011X
A. Schaefer, H. Horn, and R. Ahlrichs, J. Chem. Phys. 97, 2571 (1992). https://doi.org/10.1063/1.463096
A. Schaefer, C. Huber, and R. Ahlrichs, J. Chem. Phys. 100, 5829 (1994). https://doi.org/10.1063/1.467146
W.-M. Hoe, A. Cohen, and N. C. Handy, Chem. Phys. Lett. 341, 319 (2001). https://doi.org/10.1016/S0009-2614(01)00581-4
J. P. Perdew, K. Burke, and M. Ernzerhof, Phys. Rev. Lett. 77, 3865 (1996). https://doi.org/10.1103/PhysRevLett.77.3865
H. Paulsen, L. Duelund, H. Winkler, et al., Inorg. Chem. 40, 2201 (2001).https://doi.org/10.1021/ic000954q
M. Swart, A. R. Groenhof, A. W. Ehlers, and K. Lammertsma, J. Phys. Chem. A 108, 5479 (2004). https://doi.org/10.1021/jp049043i
M. Swart, A. W. Ehlers, and K. Lammertsma, Mol. Phys. 102, 2467 (2004).https://doi.org/10.1080/0026897042000275017
M. Swart, Inorg. Chim. Acta 360, 179 (2007). https://doi.org/10.1016/j.ica.2006.07.073
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, H. Li, H. P. Hratchian, A. F. Izmaylov, et al., Gaussian 09, Revision A.01 (Gaussian, Inc., Wallingford CT, 2009).
J. W. Ochterski, Thermochemistry in Gaussian (Gaussian, Inc., Wallingford CT, 2000).
ACKNOWLEDGMENTS
All quantum-chemical calculations were performed at the Kazan Department of the Interdepartmental Supercomputer Center of the Russian Academy of Sciences (affiliation of the Federal Research Center “Scientific Research Center for System Development,” Russian Academy of Sciences (http://www.jscc.ru)), to which the authors are grateful for technical support.
The work of D.V. Chachkov was funded by the state assignment at the Federal Research Center “Scientific Research Institute of System Analysis of Russian Academy of Sciences.”
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors have no conflicts of interest to declare that are relevant to the contents of this article.
Additional information
Translated by L. Smolina
Supplementary Information
Rights and permissions
About this article
Cite this article
Chachkov, D.V., Mikhailov, O.V. Molecular Structures of Heteroligand Macrotetracyclic Complexes of 3d Ions with Phthalocyanine and Fluoride Anion Studied by Density Functional Theory. Russ. J. Phys. Chem. 95, 310–316 (2021). https://doi.org/10.1134/S0036024421020072
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024421020072