Abstract
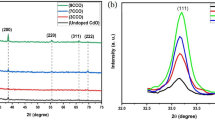
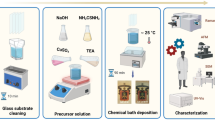
Adsorbents for the absorption of CO2 (Li2ZrO3, Li4SiO4, CaSiO3, MgO, and Li2O) are synthesized and deposited on silicon carbide. The adsorbents are effective in the range of medium (300–400°C) and high (450–750°C) temperatures, have a high capacity for the dynamic absorption of CO2, and an enhanced rate of absorption.



Similar content being viewed by others
REFERENCES
S. Kumar and S. K. Saxena, Mater. Renew. Sustain. Energy 3, 30 (2014).https://doi.org/10.1007/s40243-014-0030-9
M. J. Ramírez-Moreno, I. C. Romero-Ibarra, J. Ortiz-Landeros, and H. Pfeiffer, Alkaline and Alkaline-Earth Ceramic Oxides for CO2 Capture, Separation, and Subsequent Catalytic Chemical Conversion. https://doi.org/10.5772/57444
E. Ochoa-Fernández, M. Ronning, T. Grande, and D. Chen, Chem. Mater. 18, 1383 (2006).https://doi.org/10.1021/cm052075d
Q. Xiao, X. Tang, Y. Liu, et al., Front. Chem. Sci. Eng. 7, 297 (2013).https://doi.org/10.1007/s11705-013-1346-1
H. R. Radfarnia, High-Temperature CO2Sorbents and Application in the Sorption Enhanced Steam Reforming for Hydrogen Production (Univ. LAVAL, Quebec, Canada, 2013), p. 70.
K. B. Yi and D. O. Eriksen, Separ. Sci. Technol. 41, 283 (2006). https://doi.org/10.1080/01496390500496884
Xiao Q, Y. Liu, Y. Zhong, and W. Zhu, J. Mater. Chem. 41, 3838 (2011). https://doi.org/10.1039/C0JM03243C
M. J. Venegas, E. Fregoso-Israel, R. Eskamilla, and H. Pfeiffer, Ing. Eng. Chem. Res. 41, 2407 (2007).https://doi.org/10.1021/ie061259e
M. Kato, K. Nakagawa, K. Essaki, et al., Int. J. Appl. Ceram. Technol. 2, 467 (2005). https://doi.org/10.1111/j.1744-7402.2005.02047.x
B. K. Kishor, Y. R. Reshma, H. R. Vilash, and G. G. Abaji, Adsorpt. Sci. Technol. 30, 817 (2012).
A. Hassanzadeh and J. Abbasian, Fuel 89, 1287 (2010). https://doi.org/10.1016/j.fuel.2009.11.017
J. Fagerlund, J. Highfield, and R. Zevenhoven, RSC Adv. 2, 10380 (2012). https://doi.org/10.1039/C2RA21428H
Y. Duan and D. C. Sorescu, Phys. Rev. B 79, 014301 (2009). https://doi.org/10.1103/PhysRevB.79.014301
G. K. Lavrenchenko, A. V. Kopytin, A. I. Pyatnichko, and Yu. V. Ivanov, Tekh. Gazy, No. 1, 31 (2011).
M. Xiao, H. Liu, H. Gao, and Z. Liang, J. Chem. Thermodyn. 122, 170 (2018).https://doi.org/10.1016/j.jct.2018.03.020
P. Lang, F. Denes, and L. Hegely, Chem. Eng. Trans. 61, 1105 (2017). https://doi.org/10.3303/CET1761182
A. V. Ignatov, A. L. Tarasov, L. M. Kustov, and I. S. Portyakova, RF Patent No. 2659256, Byull. Izobret., No. 16 (2018).
E. A. Denisova, Ya. B. Baklanova, and L. G. Maksimova, RF Patent No. 2440298, Byull. Izobret., No. 2 (2012).
Q. Xiao, Y. Liu, Y. Zhong, and W. Zhu, J. Mater. Chem. 21, 3838 (2011). https://doi.org/10.1039/C0JM03243C
V. V. Kachala, L. L. Khemchyan, A. S. Kashin, N. V. Orlov, A. A. Grachev, S. S. Zalesskiy, and V. P. Ananikov, Russ. Chem. Rev. 82, 648 (2013). https://doi.org/10.1070/RC2013v082n07ABEH004413
K. Nakagawa and T. Ohashi, J. Electrochem. Soc. 145, 1344 (1998). https://doi.org/10.1149/1.1838462
K. Nakagawa and T. Ohashi, Electrochem. 67, 618 (1999).
J. Ida, R. Xiong, and Y. S. Lin, Sep. Rurif. Technol. 36, 41 (2004).https://doi.org/10.1016/S1383-5866(03)00151-5
B. N. Nair, T. Yamaguchi, H. Kawamura, et al., J. Am. Ceram. Soc. 87, 68 (2008). https://doi.org/10.1111/j.1551-2916.2004.00068.x
E. Ochoa-Fernandes, M. Ronning, T. Grande, and D. Chen, Chem. Mater. 18, 6037 (2006). https://doi.org/10.1021/cm061515d
Q. Xiao, X. Tang, Y. Liu, et al., Chem. Eng. J. 174, 231 (2011).
C. Wang, Dou B, Y. Song, et al., Ind. Eng. Chem. Res. 53, 12744 (2014).https://doi.org/10.1021/ie502042p
G. Pannocchia, M. Puccini, M. Seggiani, and S. Vitolo, Ind. Eng. Chem. Res. 46, 6696 (2007).https://doi.org/10.1021/ie0616949
M. Y. Veliz-Enriquez, G. Gonzalez, and H. Pfeiffer, J. Solid State Chem. 180, 2485 (2007). https://doi.org/10.1016/j.jssc.2007.06.023
J. Ida and Y. S. Lin, Environ. Sci. Technol. 37, 1999 (2003). https://doi.org/10.1021/es0259032
M. Khokhani, R. Khomane, and B. D. Kulkarni, J. Sol-Gel Sci. Technol. 61, 316 (2012). https://doi.org/10.1007/s10971-011-2629-y
R. B. Khomane, B. K. Sharma, S. Saha, and B. D. Kulkarni, Chem. Eng. Sci. 61, 3415 (2006).
X. Wu, Z. Wen, X. Xu, X. Wang, and J. Lin, J. Nucl. Mater. 392, 471 (2009). https://doi.org/10.1016/j.jnucmat.2009.04.010
M. J. Venegas, E. Fregoso-Israel, R. Escamilla, and H. Pfeiffer, Ind. Eng. Chem. Res. 46, 2407 (2007). https://doi.org/10.1021/ie061259e
H. Xu, W. Cheng, X. Jin, et al., Ind. Eng. Chem. Res. 52, 1886 (2013). https://doi.org/10.1021/ie301178p
C. Gauer and W. Heschel, J. Mater. Sci. 41, 2405 (2006).
P. V. Korake and A. G. Gaikwad, Front. Chem. Sci. Eng. 5, 215 (2011). https://doi.org/10.1007/s11705-010-1012-9
M. Wang and C. G. Lee, Energy Convers. Manage. 50, 636 (2009). https://doi.org/10.1016/j.enconman.2008.10.006
H. A. Mosqueda, C. Vazquez, P. Bosch, and H. Pfeiffer, Chem. Mater. 18, 2307 (2006). https://doi.org/10.1021/cm060122b
S. C. Lee, B. Y. Choi, T. J. Lee, and C. K. Ryu, Catal. Today 111, 385 (2006). https://doi.org/10.1016/j.cattod.2005.10.051
S. V. Churakov, M. Ianuzzi, and M. Parrinello, J. Phys. Chem. B 108, 11567 (2004). https://doi.org/10.1021/jp037935x
X. Zhao, G. Ji, W. Liu, et al., Chem. Eng. J. 332, 216 (2018). https://doi.org/10.1016/j.cej.2017.09.068
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by D. Kharitonov
Rights and permissions
About this article
Cite this article
Portyakova, I.S., Antipov, A.V., Mishin, I.V. et al. СО2 Adsorbents Deposited on Silicon Carbide. Russ. J. Phys. Chem. 94, 1482–1489 (2020). https://doi.org/10.1134/S0036024420070237
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024420070237