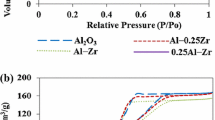
Abstract
The complete oxidation of ethane in the presence of catalysts containing 1, 3, and 5 wt % CuO deposited on four supports (zirconia, sulfated zirconia, tungstated zirconia, and La2O3-stabilized zirconia) is studied. The supports and the catalysts are characterized via BET, XRD, and thermal analysis. It is shown that 100% conversion of ethane is achieved even at a temperature of 300°C. It is found that the temperature of 100% conversion falls upon an increase in the copper content; the lowest temperature is obtained for catalysts based on unmodified zirconia. With respect to catalytic activity, the samples with the highest copper content are in the order 5%Cu/ZrO2 > 5%Cu/5%La2O3/ZrO2 > 5%Cu/15%WO3/ZrO2 > 5%Cu/5%SO4/ZrO2. The temperatures of 100% ethane conversion for these catalysts are 305, 385, 410, and 419°C, respectively.









Similar content being viewed by others
REFERENCES
F. J. Janssen and R. A. Santen, Environmental Catalysis (Imperial College Press, London, 1999).
L. Matejova, P. Topka, K. Jiratova, and O. Solcova, Appl. Catal. A: Gen. 443, 40 (2012).
M. S. Kamal, S. A. Razzak, and M. M. Hossain, Atmos. Environ. 140, 117 (2016).
L. M. Kustov, K. M. Skupov, S. S. Goryashchenko, and O. V. Masloboishchikova, Zh. Fiz. Khim. 88, 891 (2014).
A. V. Kucherov, O. P. Tkachenko, O. A. Kirichenko, et al., Top. Catal. 52, 351 (2009).
E. V. Makshina, L. V. Borovskikh, A. L. Kustov, G. N. Mazo, and B. V. Romanovskii, Russ. J. Phys. Chem. A 79, 191 (2005).
A. L. Kustov, O. P. Tkachenko, L. M. Kustov, and B. V. Romanovsky, Environ. Int. 37, 1053 (2011).
N. A. Davshan, A. L. Kustov, O. P. Tkachenko, et al., ChemCatChem 6, 1990 (2014).
T. P. Otroshchenko, A. O. Turakulova, V. A. Voblikova, L. V. Sabitova, S. V. Kutsev, and V. V. Lunin, Russ. J. Phys. Chem. A 87, 1804 (2013).
Yu. P. Semushina, S. I. Pechenyuk, L. F. Kuz’mich, and A. I. Knyazeva, Russ. J. Phys. Chem. A 91, 26 (2017).
I. Yu. Kaplin, E. S. Lokteva, E. V. Golubina, K. I. Maslakov, S. A. Chernyak, A. V. Levanov, N. E. Strokova, and V. V. Lunin, Russ. J. Phys. Chem. A 90, 2157 (2016).
K. I. Slovetskaya, A. A. Greish, M. P. Vorob’eva, and L. M. Kustov, Russ. Chem. Bull. Int. Ed. 50, 1589 (2001).
A. N. Pushkin, O. K. Gulish, D. A. Koshcheeva, and M. S. Shebanov, Russ. J. Phys. Chem. A 87, 23 (2013).
Y. Fang and Y. Guo, Chin. J. Catal. 39, 566 (2018).
A. Bialas, T. Kondratovicz, M. Drozdec, and P. Kustrowski, Catal. Today 257, 144 (2015).
G. Aguila, F. Gracia, J. Cortes, and P. Araya, Appl. Catal. B: Environ. 77, 325 (2008).
A. V. Ivanov and L. M. Kustov, Ross. Khim. Zh. 2, 21 (2000).
K. Arata, Adv. Catal. 37, 165 (1990).
A. V. Ivanov, E. G. Khelkovskaya-Sergeeva, and T. V. Vasina, Russ. Chem. Bull. 48, 1266 (1999).
M. Scheifauer, R. Grasselli, and H. Knozinger, Langmuir 14, 30 (1998).
A. L. Klyachko-Gurvich, Izv. Akad. Nauk SSSR, Otd. Khim. Nauk, No. 10, 1884 (1961).
A. Clearfield, G. P. D. Serrette, and A. H. Khazi-Syed, Catal. Today 20, 295 (1994).
O. Kirichenko, G. Kapustin, V. Nissenbaum, et al., J. Therm. Anal. Calorim. 134, 233 (2018).
B. Reddy and M. Patil, Chem. Rev. 109, 2185 (2009).
L. P. Kolmakova, O. N. Kovtun, and N. N. Dovzhenko, J. Siber. Fed. Univ., Eng. Technol. 3, 293 (2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by M. Timoshinina
Rights and permissions
About this article
Cite this article
Donyush, P.A., Kustov, A.L., Nissenbaum, V.D. et al. Ethane Oxidation in the Presence of Copper-Containing Zirconia Modified with Acid Additives. Russ. J. Phys. Chem. 93, 2140–2145 (2019). https://doi.org/10.1134/S0036024419110086
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024419110086