Abstract
A full set of the energy characteristics describing dissociation (Do(Sr–OH) = 401.8 ± 3.0 kJ/mol), ionization (Io(SrOH) = 5.372 ± 0.042 eV), and radiation power in the visible spectrum where the brightest bands with momenta Re (au) of transitions A2Π → X2Σ (2.08 ± 0.15) and B2Σ → X2Σ (1.79 ± 0.13) are located is determined for strontium monohydroxide via spectrophotometry, and from a physical model.




Similar content being viewed by others
Notes
The error in C corresponds to the uncertainty in q.
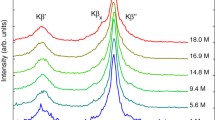
Value a = 1.1 was given for T = 2500 K in [21]. It was recalculated to the temperature of the flames of ∼2700 K used here, at which self-absorption was highest but still no more than 20%.
REFERENCES
V. N. Belyaev, Russ. J. Phys. Chem. A 90, 2188 (2016).
V. N. Belyaev and N. L. Lebedeva, Russ. J. Phys. Chem. A 86, 920 (2012).
V. N. Belyaev and N. L. Lebedeva, Russ. J. Phys. Chem. A 84, 1841 (2010).
V. N. Belyaev, N. L. Lebedeva, and K. S. Krasnov, Russ. J. Phys. Chem. A 70, 1328 (1996).
L. V. Gurvich, I. V. Veits, V. A. Medvedev, et al., Thermodynamic Properties of Individual Substances, The Reference Book, Ed. by V. P. Glushko (Nauka, Moscow, 1978–1982), Vols. 1–4 [in Russian].
Yu. N. Dmitriev, Cand. Sci. (Phys. Math.) Dissertation (Inst. High Temp. Acad. Sci. SSSR, Moscow, 1988).
P. D. Kleinschmidt, K. H. Lau, and D. L. Hildenbrand, J. Chem. Phys. 74, 653 (1981).
L. V. Gurvich, V. G. Ryabova, A. N. Khitrov, and E. M. Starovoitov, Teplofiz. Vys. Temp. 9, 290 (1971).
L. V. Gurvich, V. G. Ryabova, and A. N. Khitrov, Faraday Symp. Chem. Soc., No. 8, 83 (1973).
A. N. Khitrov, Cand. Sci. (Chem.) Dissertation (Inst. High Temp. Acad. Sci. SSSR, Moscow, 1974).
D. E. Jensen and G. A. Jones, Proc. R. Soc. London, Ser. A 364, 509 (1978).
E. Murad, J. Chem. Phys. 75, 4080 (1981).
J. van der Hurk, Tj. Hollander, and C. Th. J. Alkemade, J. Quant. Spectr. Radiat. Transfer 13, 273 (1973).
E. M. Bulewicz, C. G. James, and T. M. Sugden, Proc. R. Soc. London, Ser. A 235, 89 (1956).
M. W. Chase, Jr., J. L. Curnutt, R. A. McDonald, and A. N. Syverud, J. Phys. Chem. Ref. Data 7 (3), 793 (1978).
J. Nakagawa, R. F. Wormsbecher, and D. O. Harris, J. Mol. Spectrosc. 97, 37 (1983).
C. R. Brazier and P. F. Bernath, J. Mol. Spectrosc. 114, 163 (1985).
V. N. Belyaev, N. L. Lebedeva, K. S. Krasnov, and L. V. Gurvich, Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol. 25, 834 (1982).
P. J. Dagdigian, H. W. Cruse, and R. N. Zare, J. Chem. Phys. 60, 2330 (1974).
N. L. Lebedeva and V. N. Belyaev, Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol. 36 (6), 27 (1993).
E. Hinnov and H. Kohn, J. Opt. Soc. Am. 47, 156 (1957).
N. J. Friswell and D. R. Jenkins, Combust. Flame 19, 197 (1972).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Boltukhina
Rights and permissions
About this article
Cite this article
Belyaev, V.N. Energy Characteristics of a Strontium Monohydroxide Molecule. Russ. J. Phys. Chem. 93, 2203–2212 (2019). https://doi.org/10.1134/S0036024419110062
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024419110062