Abstract
The simplest isobaric–isothermic phase diagram for a five-component system can be considered a four-dimensional simplex (pentatope) containing 5 vertices, 10 edges, 10 triangular faces, and 5 tetrahedral hyperfaces. Adding one compound formed from r components (2 ≤ r ≤ 5) to the system partitions the simplex into 2–5 filial pentatopes, in which the neighboring pentatopes have a common tetrahedral face. It is proposed that the topology of the system be described using two graphs. The vertices of the graph of a diagram are labeled with the symbols of components and compounds, while its edges indicate which of these phases are in thermodynamic equilibrium. The vertices of the pentatope adjacency graph symbolize pentatopes, and the edge connects two adjacent pentatopes with a common tetrahedral hyperface. Knowledge of the adjacency graph allows enumeration of all elements of the diagram and determination of their relative positions in 4D space. Topological properties characteristic of phase diagrams are considered.





Similar content being viewed by others
Notes
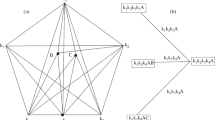
The projections of diagrams with quaternary and quinary compounds presented in Figs. 4 and 5 are isomorphic, since it is not clear whether the point of a compound is inside the tetrahedron or inside the pentatope. We can distinguish these variants from at least two projections of the diagram onto different planes. The main difference between the diagrams is reflected in the adjacency graph of the pentatopes.
REFERENCES
N. S. Kurnakov, Introduction to Physical and Chemical Analysis (Akad. Nauk SSSR, Moscow, 1940) [in Russian].
F. N. Rhines, Phase Diagrams in Metallurgy Their Development and Application (McGraw-Hill, 1956).
R. Vogel, Die heterogenen Gleichgewichte (Akad. Verlag, Leipzig, 1959).
A. M. Zakharov, State Diagrams of Quaternary Systems (Metallurgiya, Moscow, 1964) [in Russian].
V. Ya. Anosov, M. I. Ozerova, and Yu. Ya. Fialkov, Major Methods of Physicochemical Analysis (Nauka, Moscow, 1976) [in Russian].
V. I. Kosyakov, Russ. J. Inorg. Chem. 55, 1779 (2010);
Russ. J. Inorg. Chem. 55, 1786 (2010).
V. I. Kosyakov, V. A. Shestakov, E. V. Grachev, and V. Yu. Komarov, Russ. J. Inorg. Chem. 59, 1501 (2014).
V. Kosyakov, V. Shestakov, and E. V. Grachev, Match-Commun. Math. 69, 795 (2013).
D. A. Petrov, Quaternary Systems: A New Approach to Construction and Analysis (Metallurgiya, Moscow, 1991) [in Russian].
V. I. Kosyakov, V. A. Shestakov, E. V. Grachev, and V. Yu. Komarov, Russ. J. Inorg. Chem. 61, 1274 (2016).
Yu. A. Kazik, Mathematical Dictionary (Valgus, Tallinn, 1985) [in Russian].
G. F. Voronin and A. L. Voskov, Mosc. Univ. Chem. Bull. 68, 1 (2013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Polyakov
Rights and permissions
About this article
Cite this article
Kosyakov, V.I. Topology of Isobaric–Isothermic Phase Diagrams for Five-Component Systems. Russ. J. Phys. Chem. 93, 1635–1640 (2019). https://doi.org/10.1134/S0036024419090085
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024419090085