Abstract
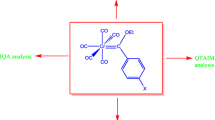
In this investigation substituent effect on the pKa values of the Cr(CO)3(para-XC6H4COOH) complexes (X = NH2, OH, H, F, Cl, CN, NO2) was demonstrated at the wB97XD/6-311G(d,p) level of theory through aqueous phase calculation. The conductor-like polarized continuum model (CPCM) was used for calculation in solution phase. The CPCM calculations were accompanied with SMD-Coulomb atomic radii. The linear correlation relationships that can be established between the calculated pKa values with Hammett constants and deprotonating energy were analyzed. Also, the atomic charges of the acidic proton were calculated through QTAIM and NBO methods and their correlations with the obtained pKa values were studied.



Similar content being viewed by others
REFERENCES
E. O. Fischer, K. Ofele, H. Essler, W. Frohlich, J. P. Mortensen, and W. Semmlinger, Z. Naturforsch. B 13, 458 (1958).
M. HudeZek and S. Toma, J. Organomet. Chem. 406, 147 (1991).
C. A. L. Mahaffy and J. Hamilton, Synth. Reactiv. Inorg. Met.-Org. Chem. 17, 849 (1987).
C. A. L. Mahaffy and J. Hamilton, Synth. Reactiv. Inorg. Met.-Org. Chem. 17, 43 (1987).
E. O. Fischer, K. Ofele, H. Essler, W. Frolich, J. P. Mortensrsen, and W. Semmlinger, Cherm. Ber. 91, 2763 (1958).
M. Ashraf and W. R. Jackson, J. Chem. Soc. Perkin II 11, 103 (1972).
M. K. Shamami, R. Ghiasi, and M. D. Asli, J. Chin. Chem. Soc. 64, 369 (2017).
H. Ghobadi, R. Ghiasi, and S. Jamehbozorgi, J. Chin. Chem. Soc. 64, 522 (2017).
R. Ghiasi, H. Pasdar, and S. Fereidoni, Russ. J. Inorg. Chem. 61, 327 (2016).
R. Ghiasi and A. Heydarbeighi, Russ. J. Inorg. Chem. 61, 985 (2016).
R. Ghiasi, H. Pasdar, and F. Irajizadeh, J. Chil. Chem. Soc 60, 2740 (2015).
A. Peikari, R. Ghiasi, and H. Pasdar, Russ. J. Phys. Chem. A 89, 250 (2015).
R. Ghiasi and E. Amini, J. Struct. Chem. 56, 1483 (2015).
M. Z. Fashami and R. Ghiasi, J. Struct. Chem. 56, 1474 (2015).
R. Ghiasi and H. Pasdar, Russ. J. Phys. Chem. A 87, 973 (2013).
R. Ghiasi and A. Boshak, J. Mex. Chem. Soc. 57, 8 (2013).
H. Pasdar and R. Ghiasi, Main Group Chem. 8, 143 (2009).
C. Hansch, A. Leo, and R. W. Taft, Chem. Rev. 97, 165 (1991).
L. P. Hammett, J. Am. Chem. Soc. 59, 96 (1937).
S. Kheirjou, A. Abedin, A. Fattahi, and M. M. Hashemi, Comput. Theor. Chem. 1027, 191 (2014).
U. A. Chaudry and P. L. A. Popelier, J. Org. Chem. 69, 233 (2004).
J. Zhang, Y. Sun, C. Mao, H. Gao, W. Zhou, and Z. Zhou, J. Mol. Struct.: THEOCHEM 906, 46 (2009).
K. C. Gross and P. G. Seybold, Int. J. Quantum Chem. 80, 1107 (2000).
G.-C. Bahram and A. Ghiami-Shomami, Comput. Theor. Chem. 1054, 71 (2015).
M. Remko, J. Bojarska, A. Remková, and W. Maniukiewicz, Comput. Theor. Chem. 1062, 50 (2015).
D. D. Perrin, B. Dempsey, and E. P. Serjeant, pK a Prediction for Organic Acids and Bases (Chapman and Hall, Cambridge, 1981).
L. P. Hammett, Physical Organic Chemistry, 2nd ed. (McGraw-Hill, New York, 1970).
C. A. Hollingsworth, P. G. Seybold, and C. M. Hadad, Int. J. Quantum Chem. 90, 1396 (2002).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalman, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, et al., Gaussian 09 (Gaussian Inc., Wallingford, CT, 2009).
P. J. Hay, J. Chem. Phys. 66, 4377 (1977).
R. Krishnan, J. S. Binkley, R. Seeger, and J. A. Pople, J. Chem. Phys. 72, 650 (1980).
A. D. McLean and G. S. Chandler, J. Chem. Phys. 72, 5639 (1980).
A. J. H. Wachters, J. Chem. Phys. 52, 1033 (1970).
J. D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys. 10, 6615 (2008).
M. Cossi, N. Rega, G. Scalmani, and V. Barone, J. Comp. Chem. 24, 669 (2003).
A. V. Marenich, C. J. Cramer, and D. G. Truhlar, J. Phys. Chem. B 113, 6378 (2009).
T. A. Keith, TK Gristmill Software (Overland Park KS, USA, 2013). http://aim.tkgristmill.com.
A. E. Reed, L. A. Curtiss, and F. Weinhold, Chem. Rev. 88, 899 (1988).
E. D. Glendening, A. E. Reed, J. E. Carpenter, and F. Weinhold, NBO Version 3.1 (Gaussian Inc., PA, USA, 1998).
J. Tomasi and M. Persico, Chem. Rev. 94, 2027 (1994).
A. Ben-Naim, Solvation Thermodynamics (Plenum, New York, 1987).
R. Gomez-Bombarelli, M. Gonzalez-Perez, M. T. Perez-Prior, E. Calle, and J. Casado, J. Org. Chem. 74, 4943 (2009).
J. Ho and M. L. Coote, Theor. Chem. Acc. 125, 3 (2010).
R. Pliego, Chem. Phys. Lett. 367, 145 (2003).
K. Murlowska and N. Sadlej-Sosnowska, J. Phys. Chem. A 109, 5590 (2005).
I. E. Charif, S. M. Mekelleche, D. Villemin, and N. Mora-Diez, J. Mol. Struct.: THEOCHEM 818, 1 (2007).
M. D. Liptak and G. C. Shields, J. Am. Chem. Soc. 123, 7314 (2001).
D. P. Dissanayake and R. Senthilnithy, J. Mol. Struct.: THEOCHEM 910, 93 (2009).
B. Nicholls and M. C. Whitin, J. Chem. Soc., 551 (1959).
C. A. Hollingsworth, P. G. Seybold, and C. M. Hadad, Int. J. Quantum Chem. 90, 1396 (2002).
R. F. W. Bader, Atoms in Molecules: A Quantum Theory (Oxford Univ. Press, Oxford, UK, 1999).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reza Ghiasi, Zamani, A. & Shamami, M.K. Theoretical Study of Substituent Effect on the pKa Values of Cr(CO)3(para-XC6H4COOH) Complexes. Russ. J. Phys. Chem. 93, 1537–1542 (2019). https://doi.org/10.1134/S0036024419080247
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024419080247