Abstract
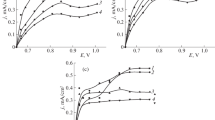
The effect of solution acidity on the electroreduction of cadmium(II) and lead(II) ions on electrodes in the presence of high-molecular water-soluble surfactants with different functional groups: metacryloyl aminophenol–acrylic acid copolymer, hydrolysate of waste from leather industry, and monoethanolamine vinyl ether acetate copolymer was studied. The kinetic and thermodynamic parameters were determined for the discharge of cadmium(II) and lead(II) ions in solutions in the absence and presence of surfactants; their values make it possible to evaluate the inhibition stage of the electrochemical reaction. It was shown that polymer surfactants can inhibit the electroreduction of cadmium(II) and lead(II) ions on like electrodes in the temperature range 298–333 K. The values of the electrochemical parameters indicate the quasi-reversible reduction of cadmium(II) and lead(II) ions; at increased surfactant concentration and increased pH values, the process becomes irreversible.
Similar content being viewed by others
REFERENCES
M. A. Loshkarev, F. I. Danilov, and V. F. Voloshin, Elektrokhimiya 7, 868 (1971).
M. K. Nauryzbaev and S. T. Shalgymbaev, Heterogeneous Chemical Reactions (Nauka, Alma-Ata, 1983) [in Russian].
E. Muller, H. Emous, H.-D. Porfler, and J. J. Lipkowsky, Electroanal. Chem. Interfacial Electrochem., No. 142, 39 (1982).
B. N. Afanas’ev and B. B. Damaskin, Elektrokhimiya 16, 280 (1980).
A. N. Frumkin and B. B. Damaskin, Adsorption and Electric Double Layer in Electrochemistry (Nauka, Moscow, 1972) [in Russian].
A. Aramata and P. Delahay, J. Phys. Chem. 68, 880 (1964).
Yu. M. Loshkarev and V. F. Vargalyuk, Elektrokhimiya 13, 1321 (1977).
E. P. Grishina, L. M. Ramenskaya, T. V. Vladimirova, and A. M. Pimenova, Russ. J. Appl. Chem. 80, 249 (2007).
V. S. Kolosnitsin and O. A. Yapryntseva, Russ. J. Appl. Chem. 77, 60 (2004).
A. Dalbanbay, A. N. Nefedov, R. A. Nurmanova, and M. K. Nauryzbayev, Chem. Bull. Kazakh. Nat. Univ., No. 87 (4), 12 (2017).
H. M. Soliman, Appl. Surf. Sci. 195, 155 (2002).
A. V. Kolesnikov, Kondens. Sredy Mezhfaz. Granitsy 18, 46 (2016).
G. K. Ziyatdinova, E. R. Ziganshina, and G. K. Budnikov, J. Anal. Chem. 67, 869 (2012).
S. I. Zhdanov, V. A. Zarinskii, and R. M.-F. Salikhdzhanova, Zh. Anal. Khim. 37, 1682 (1982).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by L. Smolina
Rights and permissions
About this article
Cite this article
Mamyrbekova, A., Kassymova, M.K. & Mamyrbekova, A. Effect of pH on the Electroreduction of Cadmium(II) and Lead(II) Ions on Electrodes in the Presence of Surfactants. Russ. J. Phys. Chem. 93, 442–446 (2019). https://doi.org/10.1134/S0036024419030129
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024419030129