Abstract
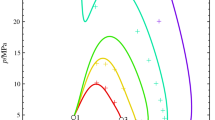
The aim of this work is the parameters test of the equation of state for hard convex body chain in predicting the critical properties of binary mixtures of n-alkanes. The predicted critical properties agree closely with the experimental results and are better than the predicted values both from the hard convex body equation of state and the Guggenheim equation of state for hard spheres. The agreement between theoretical and experimental results of the critical properties of n-alkanes appears to be improved negligibly, even if the shape of the molecule is considered. However, an improvement in the accuracy of the result is obtained with the equation of state of the hard convex body chain compared to the simple hard convex body equation of state.


Similar content being viewed by others
REFERENCES
D. Mainwaring, R. J. Sadus, and C. L. Young, Chem. Eng. Sci. 43, 45 (1987).
E. A. Guggenheim, Mol. Phys. 9, 1993 (1965).
U. Deiters, Chem. Eng. Sci. 36, 1139 (1981).
U. Deiters, Chem. Eng. Sci. 36, 1147 (1981).
U. Deiters, Chem. Eng. Sci. 37, 855 (1982).
M. S. Wertheim, J. Chem. Phys. 87, 7323 (1987).
Y. Song and J. M. Prausnitz, Macromol. 27, 441 (1994).
T. Boublik, Ber. Bunsen-Ges. 85, 1035 (1981).
T. Kihara, Rev. Mod. Phys. 25, 831 (1953).
R. J. Sadus, C. L. Young, and P. Svejda, Fluid Phase Equilib. 39, 89 (1988).
P. Svejda and F. Kohler, Ber. Bunsen-Ges. 87, 672 (1983).
F. Kohler, and P Svejda, Ber. Bunsen-Ges. 88, 101 (1984).
M. A. Siddiqi, P. Svejda, and F. Kohler, Ber. Bunsen-Ges. 88, 1176 (1983).
G. Christou, R. J. Sadus, C. L. Young, and P. Svejda, Ind. Eng. Chem. Res. 28, 481 (1989).
Y. S. Wei and R. J. Sadus, AIChE J. 46, 169 (2000).
R. J. Sadus, Mol. Phys. 97, 1279 (1999).
R. J. Sadus, AIChE J. 40, 1376 (1994).
C. P. Hicks and C. L. Young, Chem. Rev. 75, 119 (1975).
C. P. Hicks and C. L. Young, J. Chem. Soc. Faraday Trans. 73, 597 (1976).
R. Kohlen and F. Kohler, Phys. B+C (Amsterdam, Neth.) 139, 79 (1986).
G. Christou, T. Morrow, R. J. Sadus, and C. L. Young, Fluid Phase Equilib. 25, 263 (1986).
M. Christoforakas and E. U. Franck, Ber. Bunsen-Ges. 90, 780 (1986).
D. E. Mainwaring, R. J. Sadus, and C. L. Young, Fluid Phase Equilib. 42, 85 (1988).
L. W. Cummings, T. F. Stone, and U. A. Volante, Ind. Eng. Chem. 25, 728 (1933).
E. J. Partington, J. S. Rowlinson, and J. F. Weston, Trans. Faraday Soc. 56, 479 (1960).
W. B. Kay and D. Hissong, Proc. Am. Petrol. Inst. Refining Div. 47, 653 (1967).
D. Hissong and W. B. Kay, Proc. Am. Petrol. Inst. Refining Div. 48, 397 (1968).
C. P. Hicks and C. L. Young, Trans. Faraday Soc. 66, 1340 (1970).
C. P. Hicks and C. L. Young, Aust. J. Chem. 24, 2697 (1971).
C. P. Hicks and C. L. Young, J. Chem. Thermodyn. 3, 39 (1971).
S. C. Pak and W. B. Kay, Ind. Eng. Chem. Fundam. 11, 255 (1972).
C. L. Young, J. Chem. Soc. Faraday Trans. 2, 452 (1972).
A. N. Campbell and R. M. Chatterjee, Can. J. Chem. 48, 277 (1970).
I. W. Jones and J. S. Rowlinson, Trans. Faraday Soc. 59, 1702 (1963).
L. W. Jordan and W. B. Kay, Chem. Eng. Prog. Symp. Ser. 59, 46 (1963).
W. B. Kay, J. Phys. Chem. 68, 827 (1964).
A. E. H. N. Mousa, W. B. Kay, and A. Kreglewski, J. Chem. Thermodyn. 4, 301 (1972).
M. Ratzsch, Z. Phys. Chem. 243, 212 (1970).
H. Singh, F. P. Lucien, and N. R. Foster, J. Chem. Eng. Data 45, 131 (2000).
A. Diefenbacher and M. Turk, Fluid Phase Equilib. 182, 121 (2001).
J. L. Wang and R. J. Sadus, Fluid Phase Equilib. 214, 67 (2003).
N. Rai and E. J. Maginn, Faraday Discuss. 154, 53 (2012).
ACKNOWLEDGMENTS
We are indebted to S.N. Singh, A. Kapoor, S.K. Dey, A. Kumar, and A.K. Akhouri for helpful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
1The article is published in the original.
Rights and permissions
About this article
Cite this article
Akhouri, B.P., Kaur, S. Critical Properties of n-Alkanes Binary Mixtures Based on Equations of State. Russ. J. Phys. Chem. 92, 1881–1888 (2018). https://doi.org/10.1134/S0036024418100047
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024418100047