Abstract
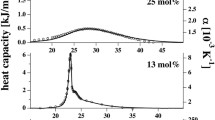
Thermodynamic properties of the cholesteryl myristate (CM) and its binary mixture CM/PCPB (p-pentylphenyl-2-chloro-4(p-pentylbenzoyl)-benzoate) are studied at the concentrations of xPCPB = 0.052 and 0.219 as a function of temperature near the cholosteric/smectic A transition. By analyzing the observed molar volume from the literature, the temperature dependences of the thermal expansion, isothermal compressibility and the difference in the specific heat are calculated and, the Pippard relations are established for those compounds close to the cholesteric/smectic A transition. Predictions of the thermodynamic quantities and the Pippard relations can be examined by the experimental measurements of the CM and its binary mixture of CM/PCPB close to the cholesteric/smectic A transition.
Similar content being viewed by others
References
G. J. Davis, R. S. Porter, and E. M. Barrall, Mol. Cryst. Liq. Cryst. 11, 319 (1970).
M. J. Janiak, C. R. Loomis, G. G. Shipley, and D. M. Small, J. Mol. Biol. 86, 325 (1974).
E. M. Barrall, R. S. Porter, and J. F. Johnson, J. Phys. Chem. 71, 895 (1967).
D. M. Small, Surface Chemistry of Biological Systems (Plenum, New York, 1970).
A. V. Galanti and R. S. Porter, J. Phys. Chem. 76, 3089 (1972).
C. W. Griffen and R. S. Porter, Mol. Cryst. Liq. Cryst. 21, 77 (1973).
R. J. Krzewski and R. S. Porter, Mol. Cryst. Liq. Cryst. 21, 99 (1973).
K. S. Kunihisa and T. Shinoda, Bull. Chem. Soc. Jpn. 48, 3506 (1973).
A. K. George and A. R. K. L. Padmini, Mol. Cryst. Liq. Cryst. 65, 217 (1981).
P. Pollmann and K. Schulte, Ber. Bunsenges, Phys. Chem. 89, 780 (1985).
H. Kishimoto, T. Iwasaki, and M. Yonese, Chem. Pharm. Bull. 34, 2698 (1986).
I. Medina, Liq. Cryst. 12, 989 (1992).
V. A. Andreev and I. V. Okinikova, Russ. Chem. Bull. 44, 1223 (1995).
L. Sabrinov, V. Lerman, Y. Turakulov, and D. Semenov, Mol. Cryst. Liq. Cryst. 366, 1 (2001).
H. Yurtseven and E. Kilit, Phys. Proc. Mugla Univ. (Turkey) 1, 597 (2008).
J. M. Schnur and D. E. Martire, Mol. Cryst. Liq. Cryst. 26, 213 (1973).
B. E. North and D. M. Small, J. Phys. Chem. 81, 723 (1977).
L. I. Mineev, I. I. Sushkin and I. G. Chistyakov, Sov. Phys. Dokl. 28, 708 (1983).
M. P. McCourt, N. Li, W. A. Pangborn, R. Miller, C. M. Weeks, and D. L. Dorset, J. Phys. Chem. 100, 9842 (1996).
S. L. Srivastava and R. Dhar, Mol. Cryst. Liq. Cryst. 366, 79 (2001).
P. H. Keyes, H. T. Weston and W. B. Daniels, Phys. Rev. Lett. 31, 628 (1973).
H. Yurtseven and S. Şen, Ann. N. Y. Acad. Sci. 1161, 416 (2009).
V. M. Semenchenko, V. M. Byankin and Y. Ya. Baskakov, Sov. Phys. Crystallogr. 20, 111 (1975).
J. Hermann, R. Sandrock, W. Spratte, and G. M. Schneider, Mol. Cryst. Liq. Cryst. Lett. 56, 183 (1980).
G. W. Gray, V. Vill, H. W. Spiess, D. Demus, and J. W. Goodby, Physical Properties of Liquid Crystals (Wiley-VCH, Weinheim, New York, etc., 2009).
H. Brown, Advances in Liquid Crystals (Academic, New York, San Francisco, London, 1976).
H. Yurtseven, B. Yolal, and O. Tari, Mol. Cryst. Liq. Cryst. 632, 97 (2016).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Yurtseven, H., Dogan, E.K. Thermodynamic Parameters of Cholesteric/Smectic A Transition in Cholesteric Myristate and Its Binary Mixture CM/PCPB. Russ. J. Phys. Chem. 92, 1208–1212 (2018). https://doi.org/10.1134/S0036024418060250
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024418060250