Abstract
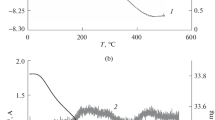
A comparative analysis of hydrogen absorption capability is performed for the first time for three types of carbon nanostructures: graphenes, oriented carbon nanotubes with graphene walls (OCNTGs), and pyrocarbon nanocrystallites (PCNs) synthesized in the pores of TRUMEM ultrafiltration membranes with mean diameters (D m) of 50 and 90 nm, using methane as the pyrolized gas. The morphology of the carbon nanostructures is studied by means of powder X-ray diffraction, X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, and transmission electron microscopy (TEM). Hydrogen adsorption is investigated via thermogravimetric analysis (TGA) in combination with mass-spectrometry. It is shown that only OCNTGs can adsorb and store hydrogen, the desorption of which under atmospheric pressure occurs at a temperature of around 175°C. Hydrogen adsorption by OCNTGs is quantitatively determined and found to be about 1.5% of their mass. Applying certain assumptions, the relationship between the mass of carbon required for the formation of single-wall OCNTGs in membrane pores and the surface area of pores is established. Numerical factor Ψ = m dep/m calc, where m dep is the actual mass of carbon deposited upon the formation of OCNTGs and mcalc is the calculated mass of carbon necessary for the formation of OCNTGs is introduced. It is found that the dependence of specific hydrogen adsorption on the magnitude of the factor has a maximum at Ψ = 1.2, and OCNTGs can adsorb and store hydrogen in the interval 0.4 to 0.6 < Ψ < 1.5 to 1.7. Possible mechanisms of hydrogen adsorption and its relationship to the structure of carbon nanoformations are examined.
Similar content being viewed by others
References
X. Sun, R. Li, B. Stansfield, J.-P. Dodelet, et al., Carbon 45, 732 (2007).
D. C. Elias, R. R. Nair, T. M. G. Mohiuddin, et al., Science 323 5914, 610 (2009).
A. Savchenco, Science 323 5914, 589 (2009).
L. Gao, X. Zhou, and Y. Ding, Chem. Phys. Lett. 434, 297 (2007).
N. N. Klimov, S. Jung, N. B. Zhitenev, et al., Science 336 6088, 1557 (2012).
H. Yang, J. Heo, S. Park, et al., Science 336 6085, 1140 (2012).
L. Britnell, A. Mishchenko, T. Georgiou, et al., Science 335 6071, 947 (2012).
J. Simon and M. Greiner, Nature 483 7389, 282 (2012).
C. F. Chen, C. H. Park, J. Horng, et al., Nature 471 7340, 617 (2011).
A. K. Ray, R. K. Sahu, V. Rajinikanth, et al., Carbon 50, 4123 (2012).
H. Hao, P. Liu, J. Tang, et al., Carbon 50, 4103 (2012).
C. Zhang, J. Li, E. Liu, et al., Carbon 50, 3513 (2012).
C. Wu, F. Li, Y. Zhang, et al., Carbon 50, 3622 (2012).
A. Hagen, G. Moos, V. Talalaev, et al., Appl. Phys. A 78, 1137 (2004).
N. Behabtu, C. C. Young, D. E. Tsentalovich, et al., Science 339 6116, 182 (2013).
J. Baringhaus, F. Edler, C. Tegenkamp, et al., Nature 506 7488, 349 (2014).
A. F. Young, J. D. Sanchez-Yamagishi, B. Hunt, et al., Nature 505 7484, 528 (2014).
N. A. Buang, F. Ismail, and M. Z. Othman, Fullerenes, Nanotubes, Carbon Nanostruct. 22, 307 (2014).
A. P. Soldatov and O. P. Parenago, Dokl. Chem. 421, 187 (2008).
A. P. Soldatov, M. V. Tsodikov, V. Yu. Bichkov, et al., Int. J. Hydrogen Energy 36, 1264 (2011).
A. P. Soldatov, M. V. Tsodikov, O. P. Parenago, and V. V. Teplyakov, Russ. J. Phys. Chem. A 84, 2102 (2010).
A. P. Soldatov, V. V. Berezkin, I. V. Gontar’, G. N. Evtyugina, and O. P. Parenago, Russ. J. Phys. Chem. A 88, 990 (2008).
E. I. Shkol’nikov, I. B. Elkina, and V. V. Volkov, RF Patent No. 2141642 (1999).
A. P. Soldatov, I. A. Rodionova, and O. P. Parenago, Russ. J. Phys. Chem. A 80, 418 (2006).
M. Shiraishi, T. Takenobu, H. Kataura, et al., Appl. Phys. A 78, 947 (2004).
G. Q. Ning, F. Wei, G. H. Luo, et al., Appl. Phys. A 78, 955 (2004).
E. Poirier, R. Chahine, P. Benard, et al., Appl. Phys. A 78, 961 (2004).
C. Liu, Y. Y. Fan, M. Liu, et al., Science 286, 1127 (1999).
Y. M. Cheng, Q. Y. Yang, and C. Liu, Carbon 39, 1447 (2001).
H-J. Shin, S. Clair, Y. Kim, et al., Nature Nanotechnol. 4, 567 (2009).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.P. Soldatov, A.N. Kirichenko, E.V. Tat’yanin, 2016, published in Zhurnal Fizicheskoi Khimii, 2016, Vol. 90, No. 7, pp. 1038–1046.
Rights and permissions
About this article
Cite this article
Soldatov, A.P., Kirichenko, A.N. & Tat’yanin, E.V. Hydrogen adsorption in the series of carbon nanostructures: Graphenes–graphene nanotubes–nanocrystallites. Russ. J. Phys. Chem. 90, 1419–1426 (2016). https://doi.org/10.1134/S0036024416070293
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024416070293